Abstract
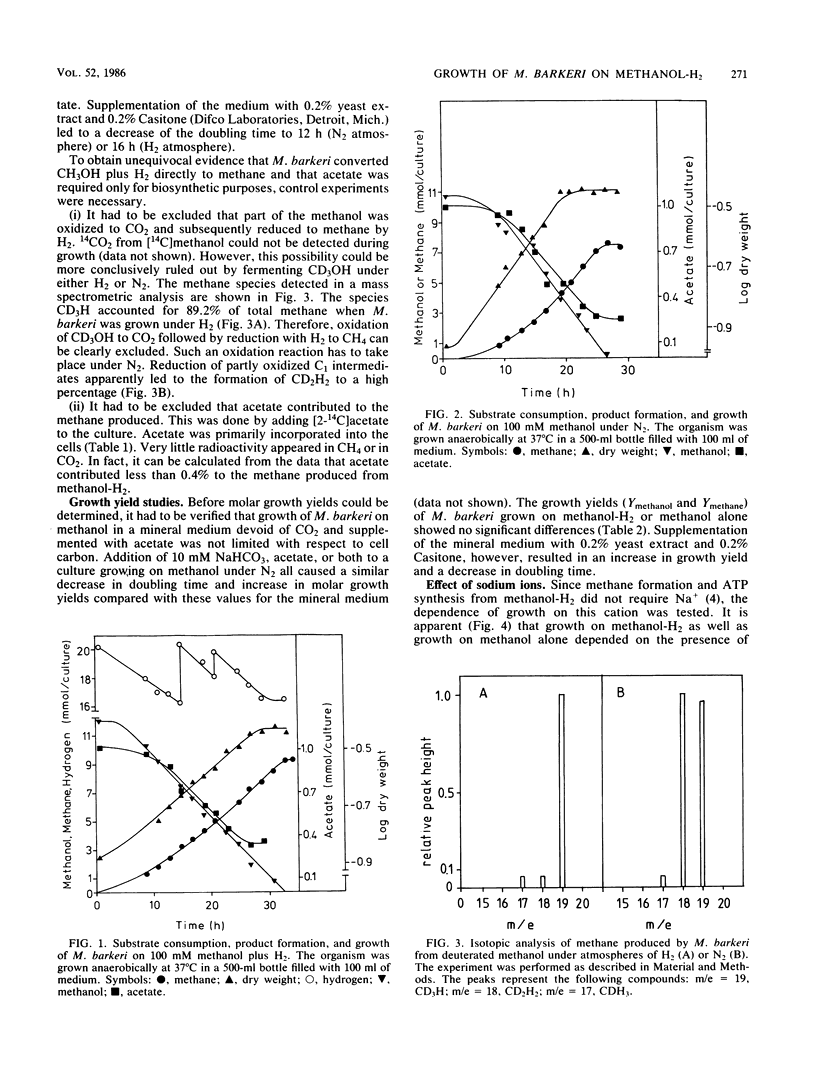
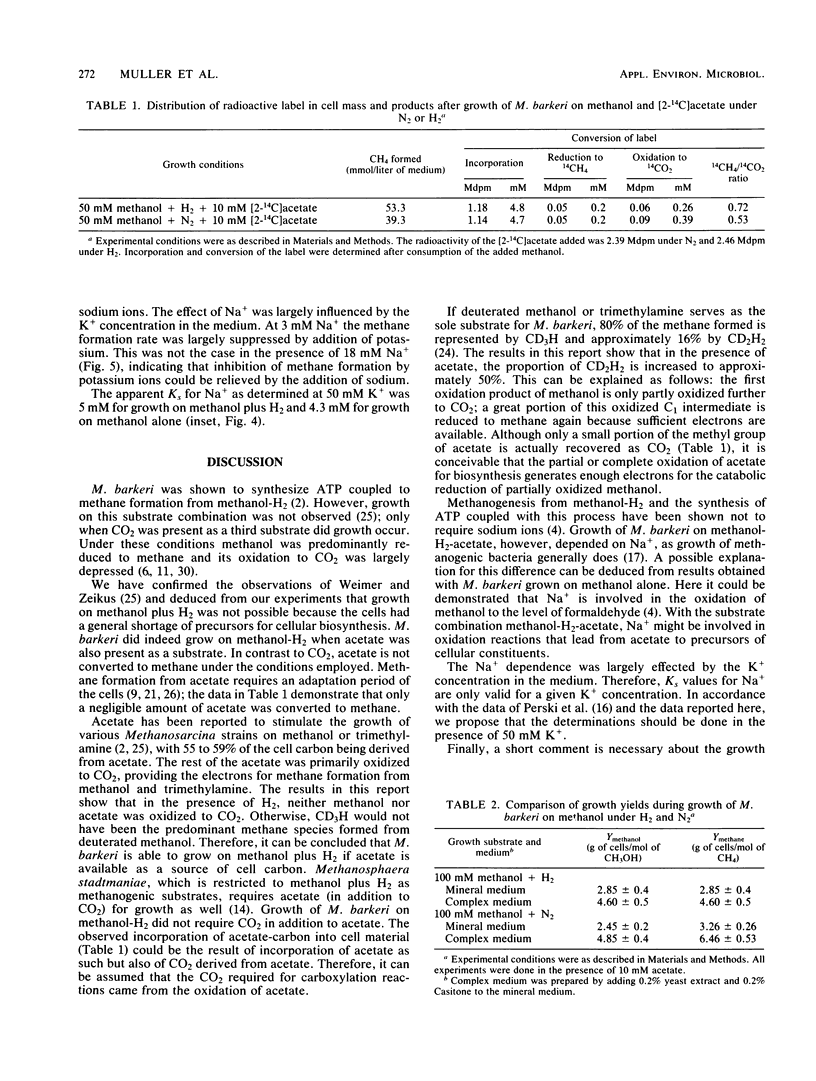
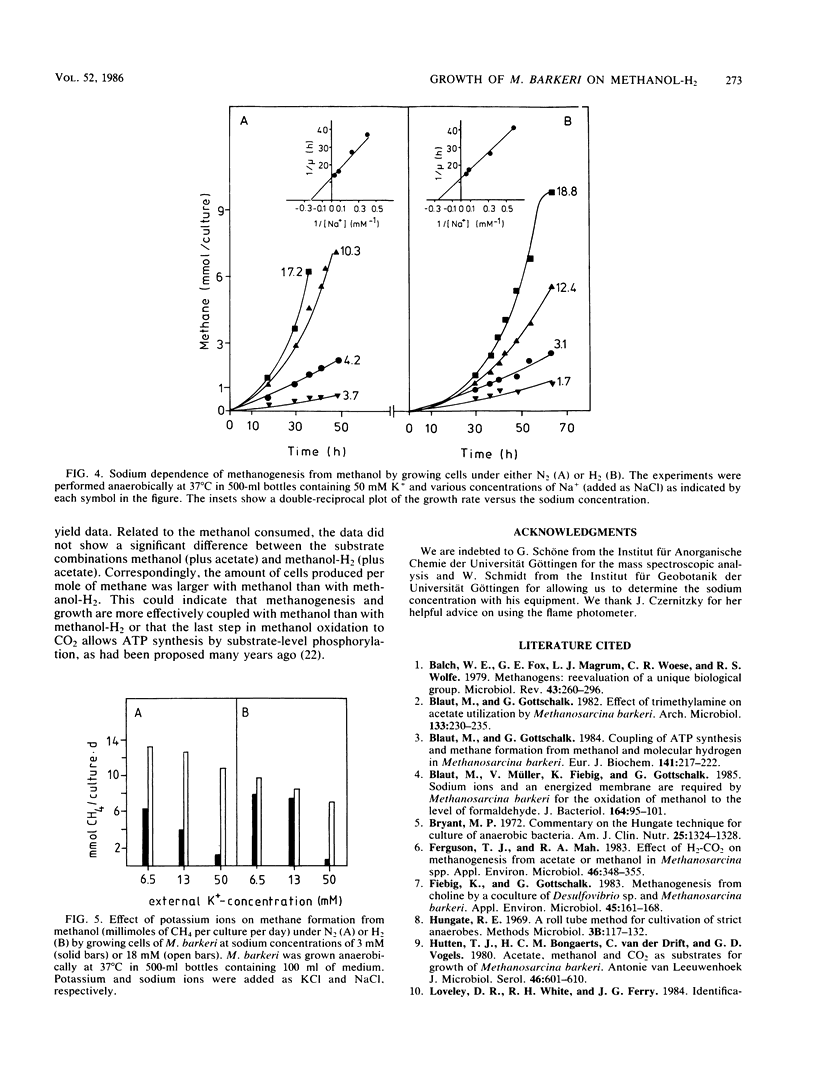
Methanosarcina barkeri grew on methanol plus H2. Both substrates were consumed in equimolar amounts. Growth was strictly dependent on the presence of acetate, which was required for the biosynthesis of cellular constituents. Only about 0.4% of the methane produced originated from acetate. By using deuterated methanol, it was demonstrated that methanogenesis from this compound under H2 did not occur via oxidation of methanol to CO2 and subsequent reduction but by direct reduction with H2. Growth yields with methanol plus H2 and with methanol alone were not significantly different: 2.8 g of cells per mol of methanol in mineral medium and 4.6 g of cells per mol of methanol in complex medium, respectively. Growth of M. barkeri on methanol plus H2 depended strictly on the presence of sodium ions in the medium. In the presence of 50 mM K+ the Ks for Na+ was 5 mM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaut M., Gottschalk G. Coupling of ATP synthesis and methane formation from methanol and molecular hydrogen in Methanosarcina barkeri. Eur J Biochem. 1984 May 15;141(1):217–222. doi: 10.1111/j.1432-1033.1984.tb08178.x. [DOI] [PubMed] [Google Scholar]
- Blaut M., Müller V., Fiebig K., Gottschalk G. Sodium ions and an energized membrane required by Methanosarcina barkeri for the oxidation of methanol to the level of formaldehyde. J Bacteriol. 1985 Oct;164(1):95–101. doi: 10.1128/jb.164.1.95-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Ferguson T. J., Mah R. A. Effect of H(2)-CO(2) on Methanogenesis from Acetate or Methanol in Methanosarcina spp. Appl Environ Microbiol. 1983 Aug;46(2):348–355. doi: 10.1128/aem.46.2.348-355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig K., Gottschalk G. Methanogenesis from Choline by a Coculture of Desulfovibrio sp. and Methanosarcina barkeri. Appl Environ Microbiol. 1983 Jan;45(1):161–168. doi: 10.1128/aem.45.1.161-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten T. J., Bongaerts H. C., van der Drift C., Vogels G. D. Acetate, methanol and carbon dioxide as substrates for growth of Methanosarcina barkeri. Antonie Van Leeuwenhoek. 1980;46(6):601–610. doi: 10.1007/BF00394016. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol. 1985 Mar;141(2):116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Oxidation of hydrogen and reduction of methanol to methane is the sole energy source for a methanogen isolated from human feces. J Bacteriol. 1983 Feb;153(2):1051–1055. doi: 10.1128/jb.153.2.1051-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S., Wolfe R. S. Methyl-coenzyme M, an intermediate in methanogenic dissimilation of C1 compounds by Methanosarcina barkeri. J Bacteriol. 1980 Feb;141(2):728–734. doi: 10.1128/jb.141.2.728-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Growth and methanogenesis by Methanosarcina strain 227 on acetate and methanol. Appl Environ Microbiol. 1978 Dec;36(6):870–879. doi: 10.1128/aem.36.6.870-879.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Methane fermentation. Annu Rev Microbiol. 1967;21:121–142. doi: 10.1146/annurev.mi.21.100167.001005. [DOI] [PubMed] [Google Scholar]
- Walther R., Fahlbusch K., Sievert R., Gottschalk G. Formation of trideuteromethane from deuterated trimethylamine or methylamine by Methanosarcina barkeri. J Bacteriol. 1981 Oct;148(1):371–373. doi: 10.1128/jb.148.1.371-373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate metabolism in Methanosarcina barkeri. Arch Microbiol. 1978 Nov 13;119(2):175–182. doi: 10.1007/BF00964270. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. One carbon metabolism in methanogenic bacteria. Cellular characterization and growth of Methanosarcina barkeri. Arch Microbiol. 1978 Oct 4;119(1):49–57. doi: 10.1007/BF00407927. [DOI] [PubMed] [Google Scholar]
- Wolfe R. S. The Sixth A.J. Kluyver Memorial Lecture. Methanogens: a surprising microbial group. Antonie Van Leeuwenhoek. 1979;45(3):353–364. doi: 10.1007/BF00443275. [DOI] [PubMed] [Google Scholar]
- Wolin E. A., Wolfe R. S., Wolin M. J. Viologen dye inhibition of methane formation by Methanobacillus omelianskii. J Bacteriol. 1964 May;87(5):993–998. doi: 10.1128/jb.87.5.993-998.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976 Dec 10;194(4270):1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meijden P., Heythuysen H. J., Pouwels A., Houwen F., van der Drift C., Vogels G. D. Methyltransferases involved in methanol conversion by Methanosarcina barkeri. Arch Microbiol. 1983 Jun;134(3):238–242. doi: 10.1007/BF00407765. [DOI] [PubMed] [Google Scholar]


