Abstract
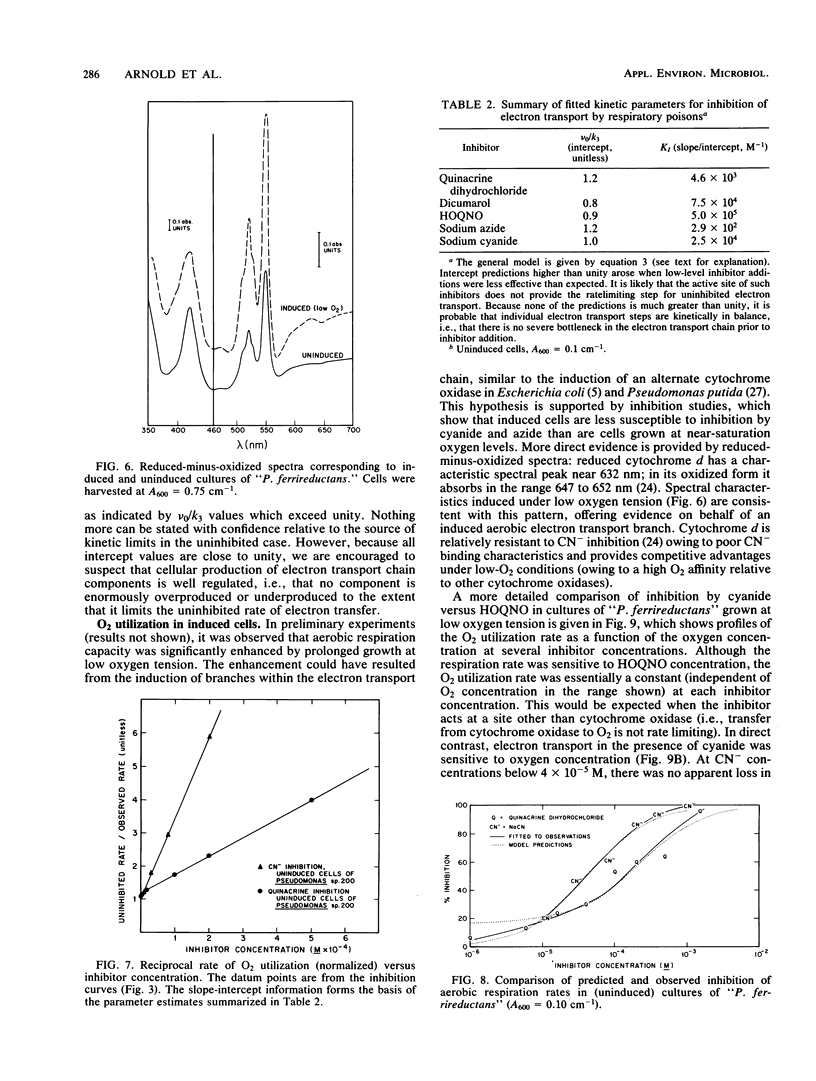
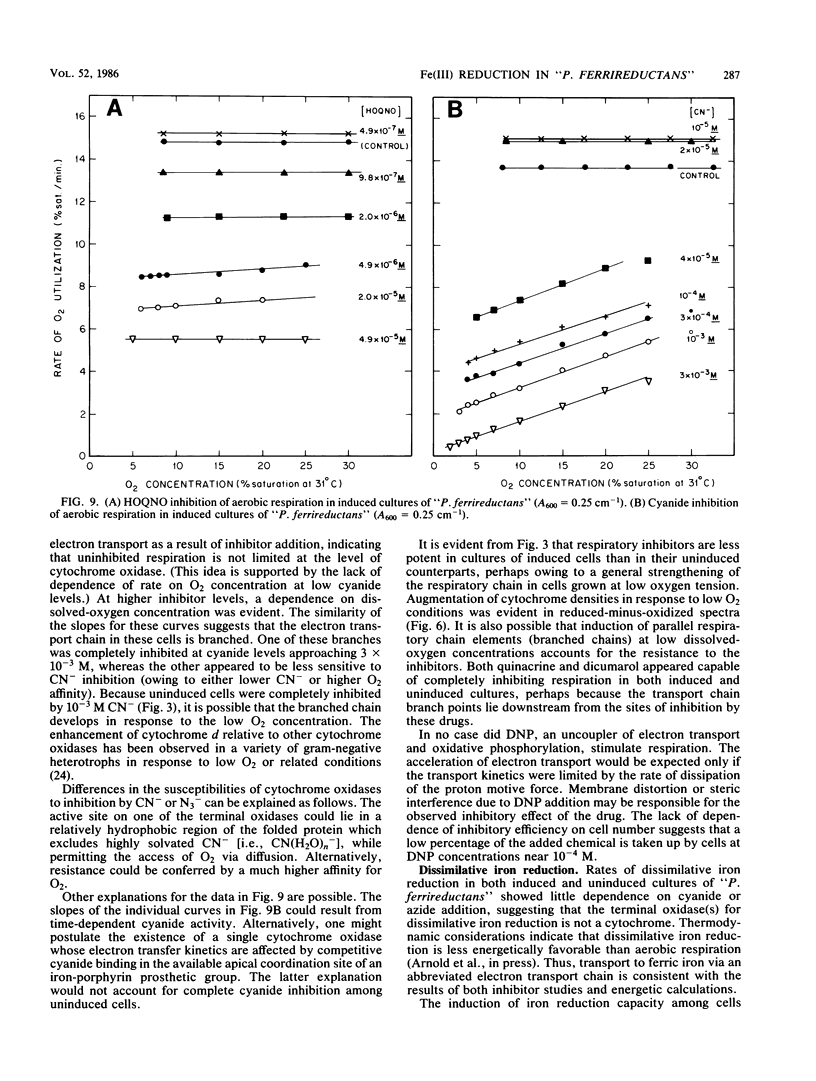
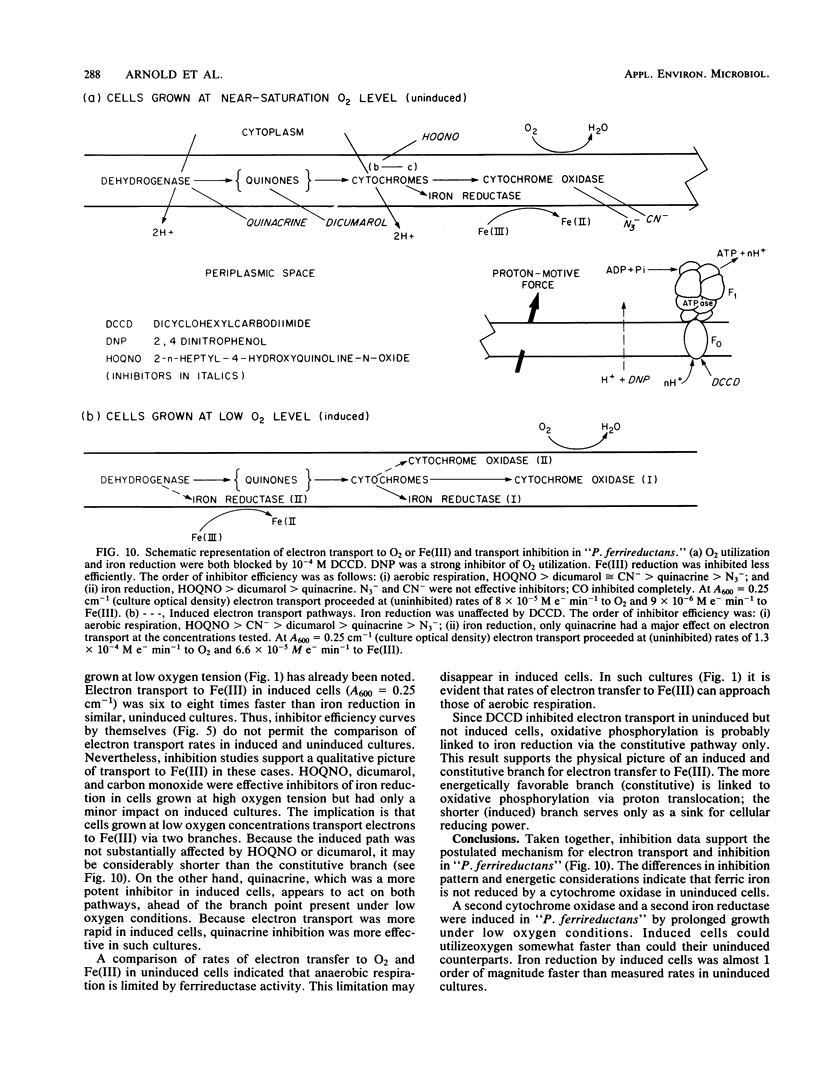
Aerobic respiration and dissimilative iron reduction were studied in pure, batch cultures of Pseudomonas sp. strain 200 ("Pseudomonas ferrireductans"). Specific respiratory inhibitors were used to identify elements of electron transport chains involved in the reduction of molecular oxygen and Fe(III). When cells were grown at a high oxygen concentration, dissimilative iron reduction occurred via an abbreviated electron transport chain. The induction of alternative respiratory pathways resulted from growth at low oxygen tension (less than 0.01 atm [1 atm = 101.29 kPa]). Induced cells were capable of O2 utilization at moderately increased rates; dissimilative iron reduction was accelerated by a factor of 6 to 8. In cells grown at low oxygen tension, dissimilative iron reduction appeared to be uncoupled from oxidative phosphorylation. Models of induced and uninduced electron transport chains, including a mathematical treatment of chemical inhibition within the uninduced, aerobic electron transport system, are presented. In uninduced cells respiring anaerobically, electron transport was limited by ferrireductase activity. This limitation may disappear among induced cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D., Gustafson J. Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl Environ Microbiol. 1976 Oct;32(4):567–571. doi: 10.1128/aem.32.4.567-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorse W. C., Ehrlich H. L. Electron transport components of the MnO2 reductase system and the location of the terminal reductase in a marine Bacillus. Appl Environ Microbiol. 1976 Jun;31(6):977–985. doi: 10.1128/aem.31.6.977-985.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle P. C., McCarty R. E. How cells make ATP. Sci Am. 1978 Mar;238(3):104-17, 121-3. doi: 10.1038/scientificamerican0378-104. [DOI] [PubMed] [Google Scholar]
- Kashket E. R. Stoichiometry of the H+-ATPase of growing and resting, aerobic Escherichia coli. Biochemistry. 1982 Oct 26;21(22):5534–5538. doi: 10.1021/bi00265a024. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The mechanism of action of DNP on phospholipid bilayer membranes. J Membr Biol. 1972;9(4):361–372. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuekwe C. O., Westlake D. W., Cook F. D. Effect of nitrate on reduction of ferric iron by a bacterium isolated from crude oil. Can J Microbiol. 1981 Jul;27(7):692–697. doi: 10.1139/m81-107. [DOI] [PubMed] [Google Scholar]
- Obuekwe C. O., Westlake D. W. Effects of medium composition on cell pigmentation, cytochrome content, and ferric iron reduction in a Pseudomonas sp. isolated from crude oil. Can J Microbiol. 1982 Aug;28(8):989–992. doi: 10.1139/m82-148. [DOI] [PubMed] [Google Scholar]
- Poole R. K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- Sweet W. J., Peterson J. A. Changes in cytochrome content and electron transport patterns in Pseudomonas putida as a function of growth phase. J Bacteriol. 1978 Jan;133(1):217–224. doi: 10.1128/jb.133.1.217-224.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troshanov E. P. Mikroorganizmy, vosstanavlivaiushchie zhelezo i marganets v rudonosnykh ozerakh karel'skogo peresheika. Mikrobiologiia. 1968 Sep-Oct;37(5):934–940. [PubMed] [Google Scholar]
- White D. C., Sinclair P. R. Branched electron-transport systems in bacteria. Adv Microb Physiol. 1971;5:173–211. doi: 10.1016/s0065-2911(08)60407-5. [DOI] [PubMed] [Google Scholar]



