Abstract
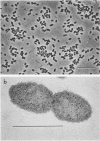
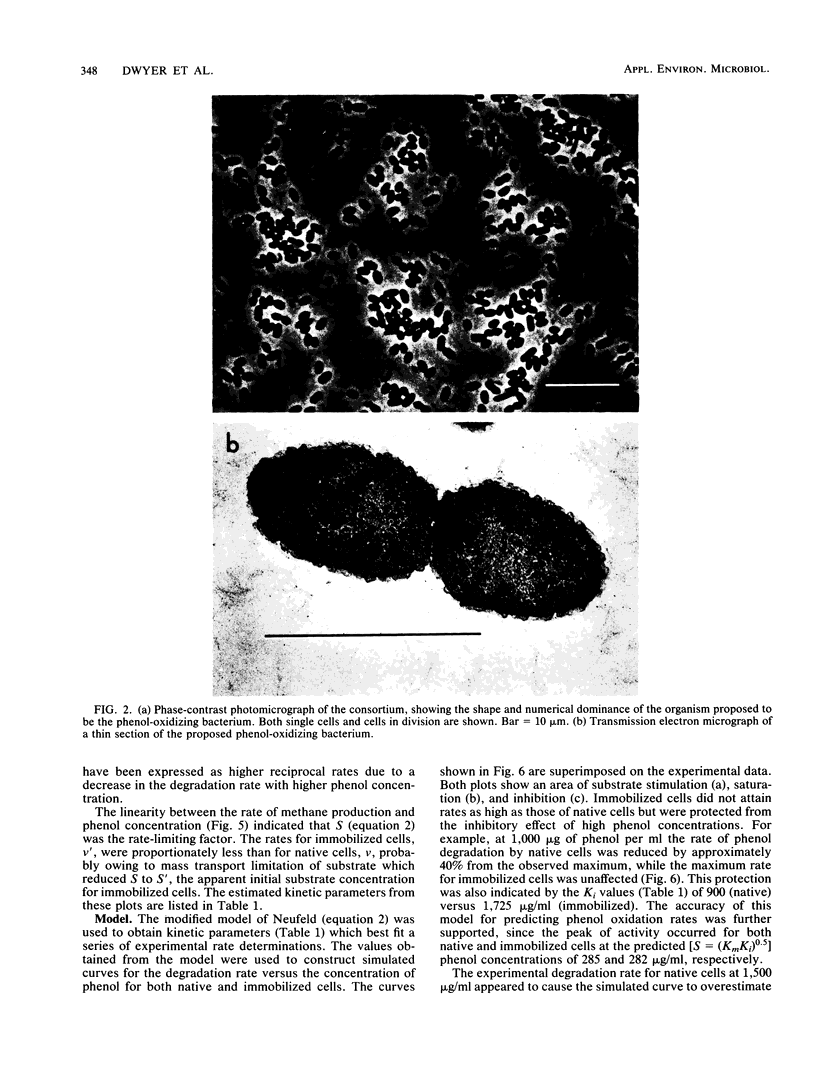
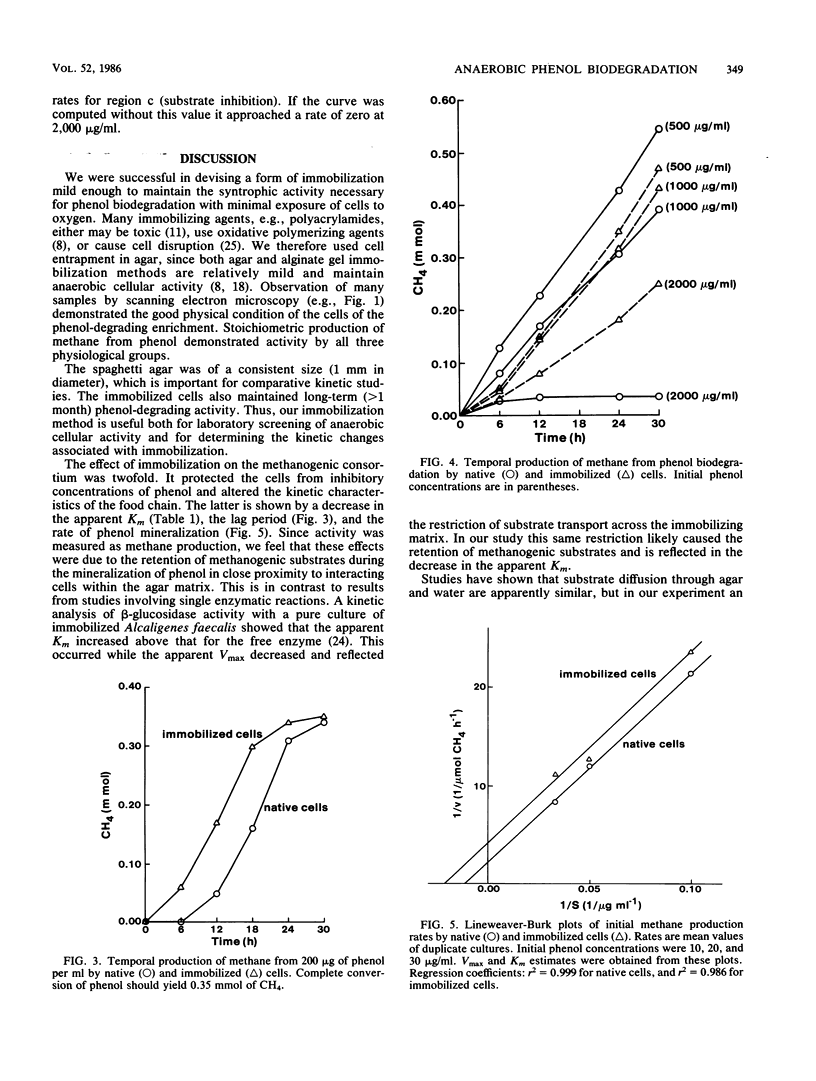
A phenol-degrading methanogenic enrichment was successfully immobilized in agar as shown by the stoichiometric conversion of phenol to CH4 and CO2. The enrichment contained members of three physiological groups necessary for the syntrophic mineralization of phenol: a phenol-oxidizing bacterium, a Methanothrix-like bacterium, and an H2-utilizing methanogen. The immobilization technique resulted in the cells being embedded in a long, thin agar strand (1 mm in diameter by 2 to 50 cm in length) that resembled spaghetti. Immobilization had three effects as shown by a comparative kinetic analysis of phenol degradation by free versus immobilized cells. (i) The maximum rate of degradation was reduced from 14.8 to 10.0 μg of phenol per h; (ii) the apparent Km for the overall reaction was reduced from 90 to 46 μg of phenol per ml, probably because of the retention of acetate, H2 and CO2 in the proximity of immobilized methanogens; and (iii) the cells were protected from substrate inhibition caused by high concentrations of phenol, which increased the apparent Ki value from 900 to 1,725 μg of phenol per ml. Estimates for the kinetic parameters Km, Ki, and Vmax were used in a modified substrate inhibition model that simulated rates of phenol degradation for given phenol concentrations. The simulated rates were in close agreement with experimentally derived rates for both stimulatory and inhibitory concentrations of phenol.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barik S., Brulla W. J., Bryant M. P. PA-1, a Versatile Anaerobe Obtained in Pure Culture, Catabolizes Benzenoids and Other Compounds in Syntrophy with Hydrogenotrophs, and P-2 plus Wolinella sp. Degrades Benzenoids. Appl Environ Microbiol. 1985 Aug;50(2):304–310. doi: 10.1128/aem.50.2.304-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards V. H. The influence of high substrate concentrations on microbial kinetics. Biotechnol Bioeng. 1970 Sep;12(5):679–712. doi: 10.1002/bit.260120504. [DOI] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. General method for determining anaerobic biodegradation potential. Appl Environ Microbiol. 1984 Apr;47(4):850–857. doi: 10.1128/aem.47.4.850-857.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtísek V., Jirků V. Immobilized cells. Folia Microbiol (Praha) 1983;28(4):309–340. doi: 10.1007/BF02879563. [DOI] [PubMed] [Google Scholar]