Abstract
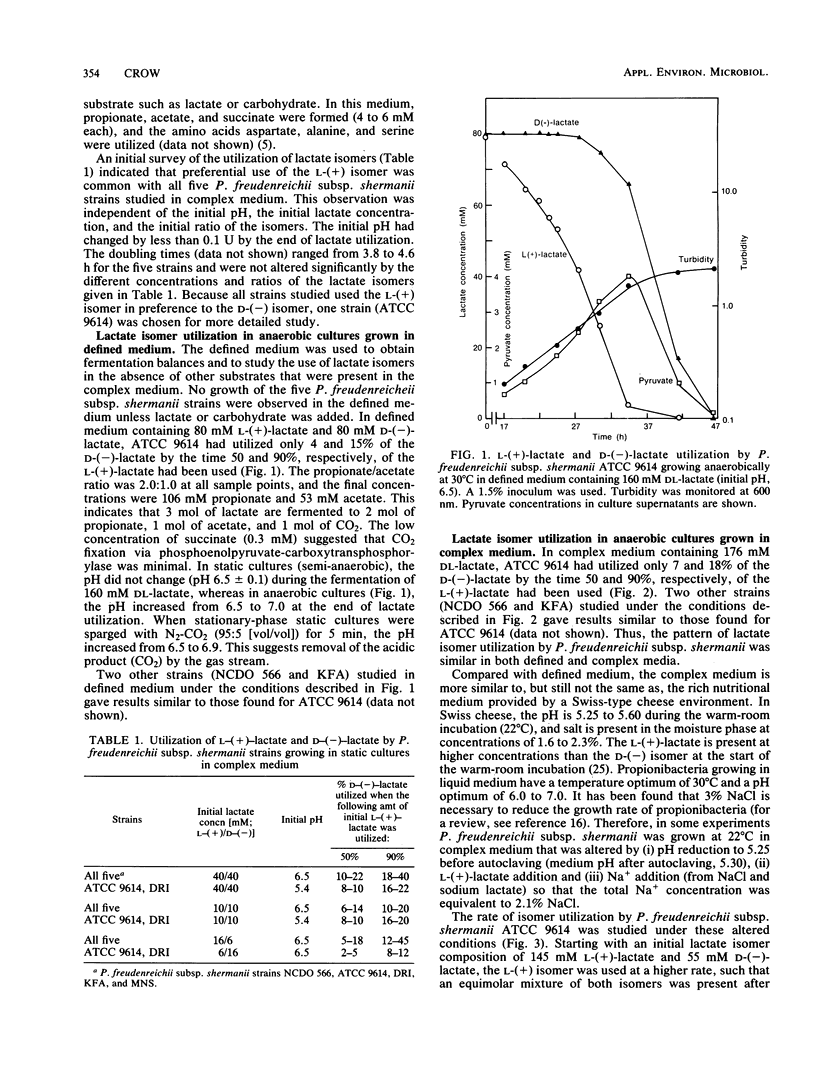
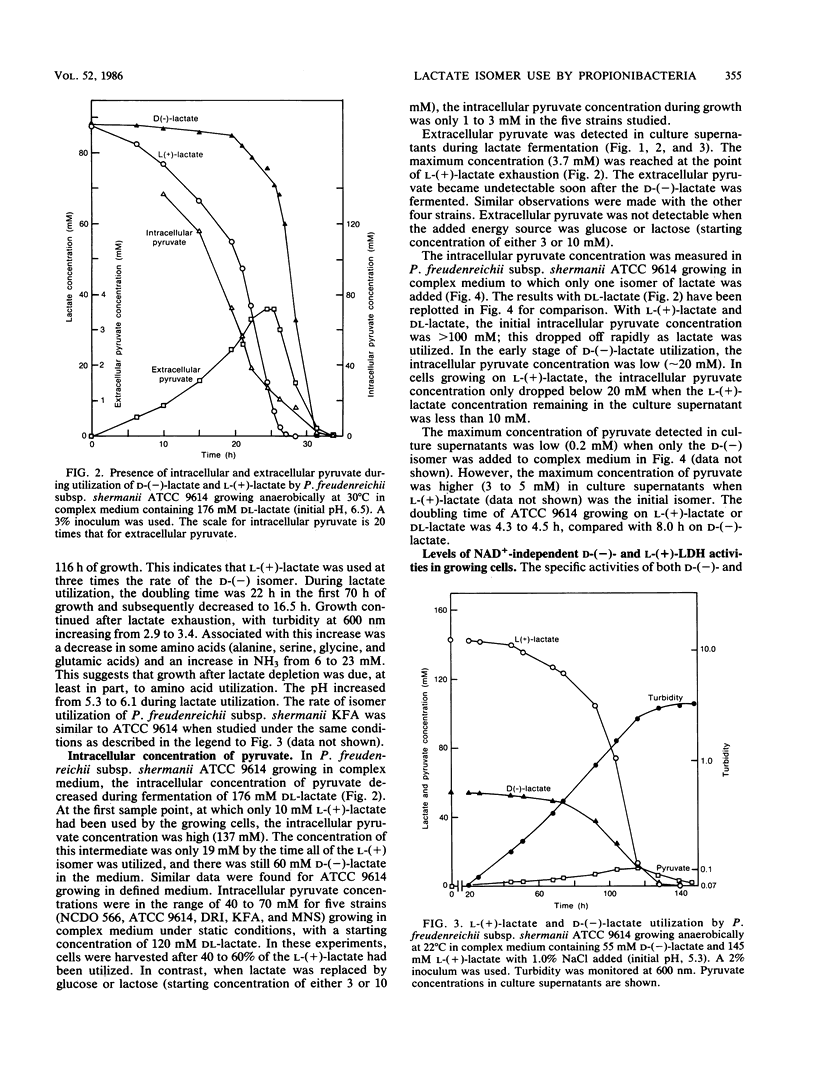
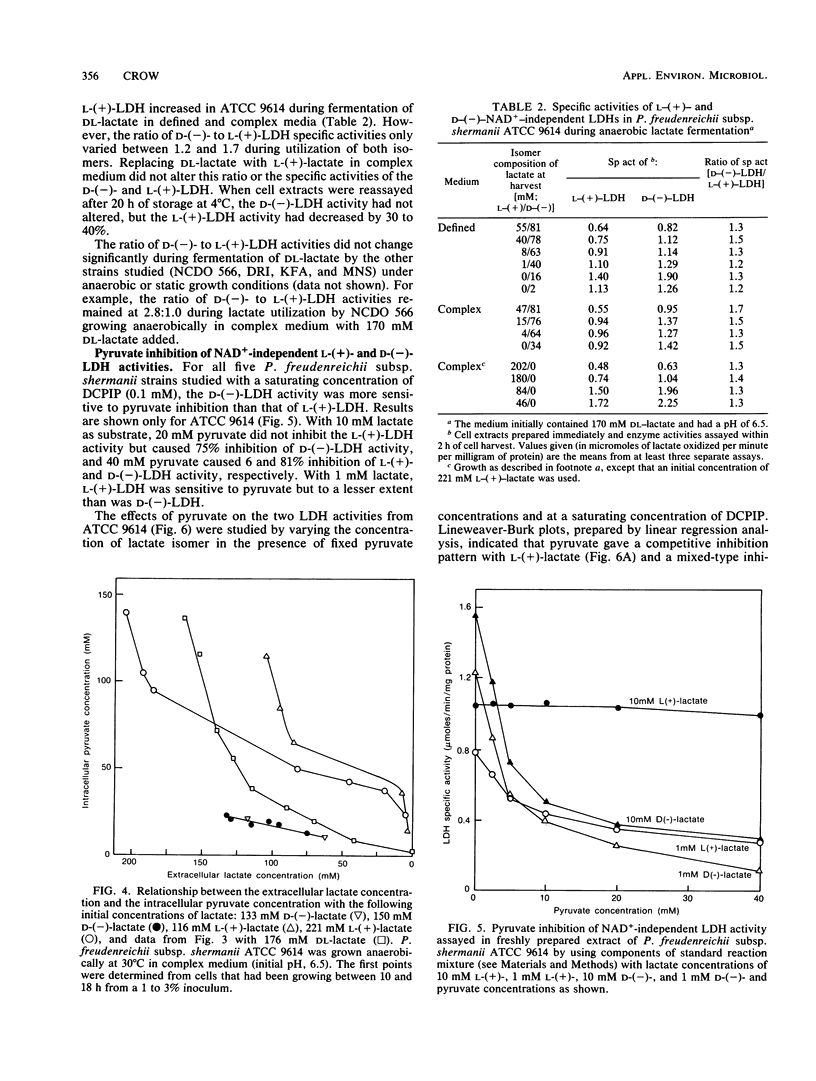
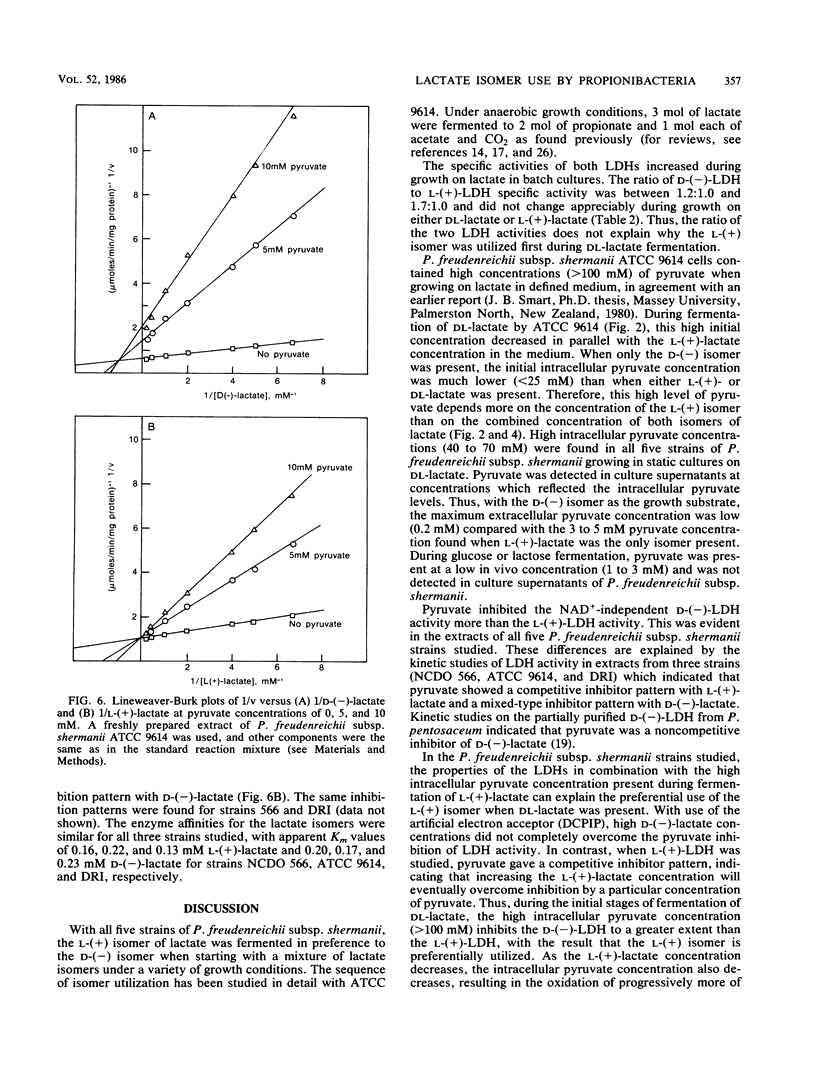
Five strains of Propionibacterium freudenreichii subsp. shermanii utilized the l-(+) isomer of lactate at a faster rate than they did the d-(−) isomer when grown with a mixture of lactate isomers under a variety of conditions. ATCC 9614, grown anaerobically in defined medium containing 160 mM dl-lactate, utilized only 4 and 15% of the d-(−)-lactate by the time 50 and 90%, respectively, of the l-(+)-lactate was used. The intracellular pyruvate concentration was high (>100 mM) in the initial stages of lactate utilization, when either dl-lactate or the l-(+) isomer was the starting substrate. The concentration of this intermediate dropped during dl-lactate fermentation such that when only d-(−)-lactate remained, the concentration was <20 mM. When only the d-(−) isomer was initially present, a similar relatively low concentration of intracellular pyruvate was present, even at the start of lactate utilization. The NAD+-independent lactate dehydrogenase activities in extracts showed different kinetic properties with regard to pyruvate inhibition, depending upon the lactate isomer present. Pyruvate gave a competitive inhibitor pattern with l-(+)-lactate and a mixed-type inhibitor pattern with d-(−)-lactate. It is suggested that these properties of the lactate dehydrogenases and the intracellular pyruvate concentrations explain the preferential use of the l-(+) isomer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Alemohammad M. M., Knowles C. J. Osmotically induced volume and turbidity changes of Escherichia coli due to salts, sucrose and glycerol, with particular reference to the rapid permeation of glycerol into the cell. J Gen Microbiol. 1974 May;82(1):125–142. doi: 10.1099/00221287-82-1-125. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cook A. M., Fewson C. A. Evidence for specific transport mechanisms for aromatic compounds in bacterium N.C.I.B. 8250. Biochim Biophys Acta. 1972 Dec 1;290(1):384–388. doi: 10.1016/0005-2736(72)90081-8. [DOI] [PubMed] [Google Scholar]
- Crow V. L. Metabolism of Aspartate by Propionibacterium freudenreichii subsp. shermanii: Effect on Lactate Fermentation. Appl Environ Microbiol. 1986 Aug;52(2):359–365. doi: 10.1128/aem.52.2.359-365.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce A. M., Crow V. L., Thomas T. D. Regulation of product formation during glucose or lactose limitation in nongrowing cells of Streptococcus lactis. Appl Environ Microbiol. 1984 Aug;48(2):332–337. doi: 10.1128/aem.48.2.332-337.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINARI R., LARA F. J. The lactic dehydrogenase of Propionibacterium pentosaceum. Biochem J. 1960 Apr;75:57–65. doi: 10.1042/bj0750057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard G. G., Asmundson R. V. Aerobic electron transport in Propionibacterium shermanii. Effects of cyanide. Arch Microbiol. 1980 Jun;126(2):167–173. doi: 10.1007/BF00511223. [DOI] [PubMed] [Google Scholar]
- Sone N. The redox reactions in propionic acid fermantation. I. Occurrence and nature of an electron transfer system in Propionibacterium arabinosum. J Biochem. 1972 Jun;71(6):931–940. doi: 10.1093/oxfordjournals.jbchem.a129864. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Turner K. W., Crow V. L. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J Bacteriol. 1980 Nov;144(2):672–682. doi: 10.1128/jb.144.2.672-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G. Metabolic cycles in the fermentation by propionic acid bacteria. Curr Top Cell Regul. 1981;18:255–287. doi: 10.1016/b978-0-12-152818-8.50021-9. [DOI] [PubMed] [Google Scholar]
- de Vries W., Wijck-Kapteijn W. M., Stouthamer A. H. Influence of oxygen on growth, cytochrome synthesis and fermentation pattern in propionic acid bacteria. J Gen Microbiol. 1972 Aug;71(3):515–524. doi: 10.1099/00221287-71-3-515. [DOI] [PubMed] [Google Scholar]
- de Vries W., van Wyck-Kapteyn W. M., Stouthamer A. H. Generation of ATP during cytochrome-linked anaerobic electron transport in propionic acid bacteria. J Gen Microbiol. 1973 May;76(1):31–41. doi: 10.1099/00221287-76-1-31. [DOI] [PubMed] [Google Scholar]


