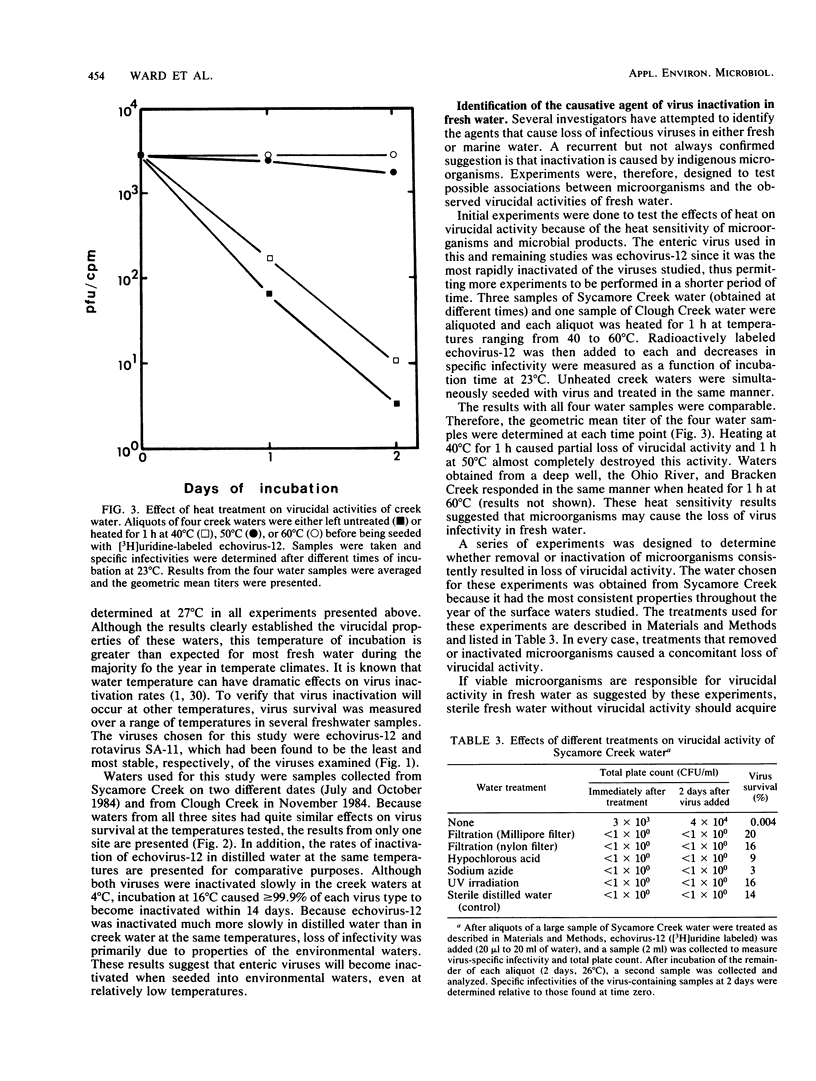
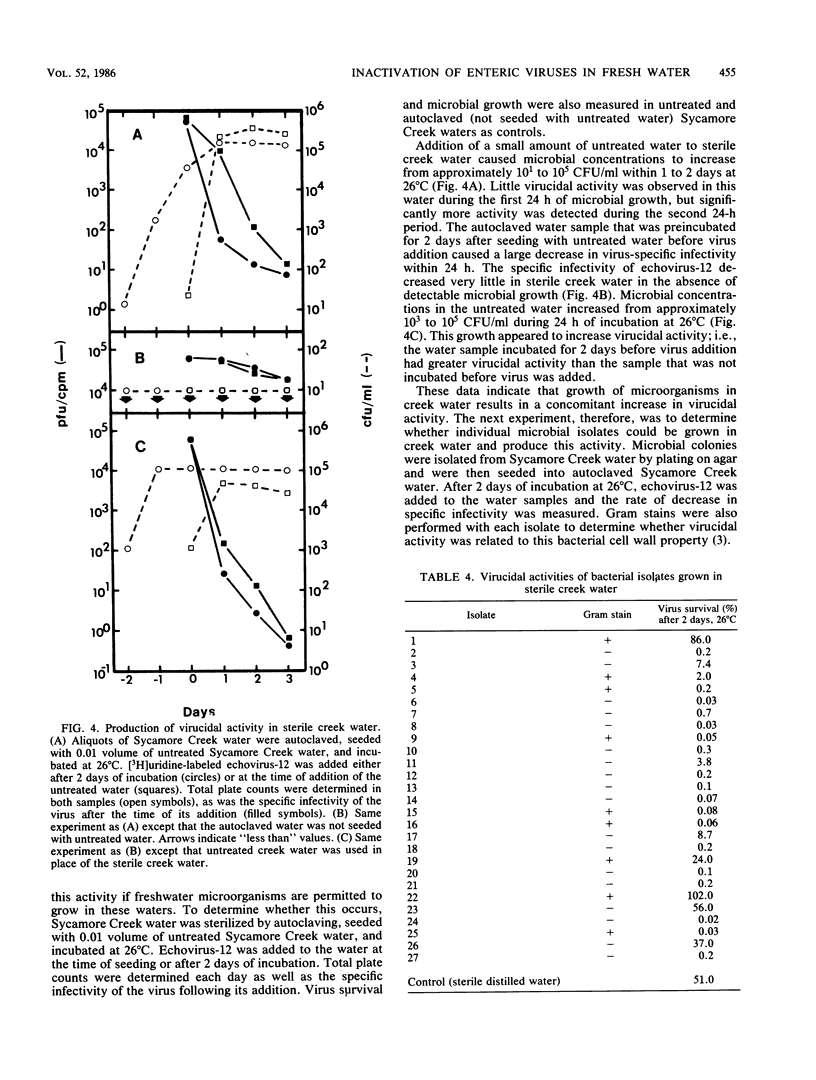
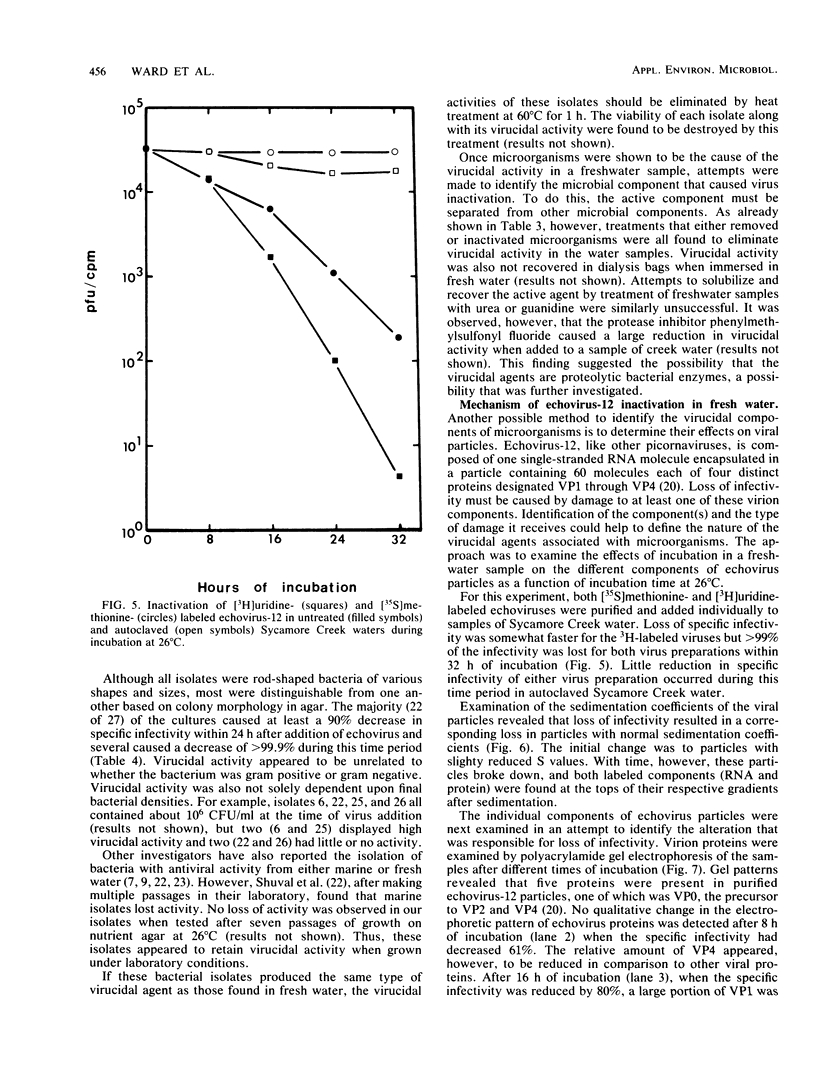
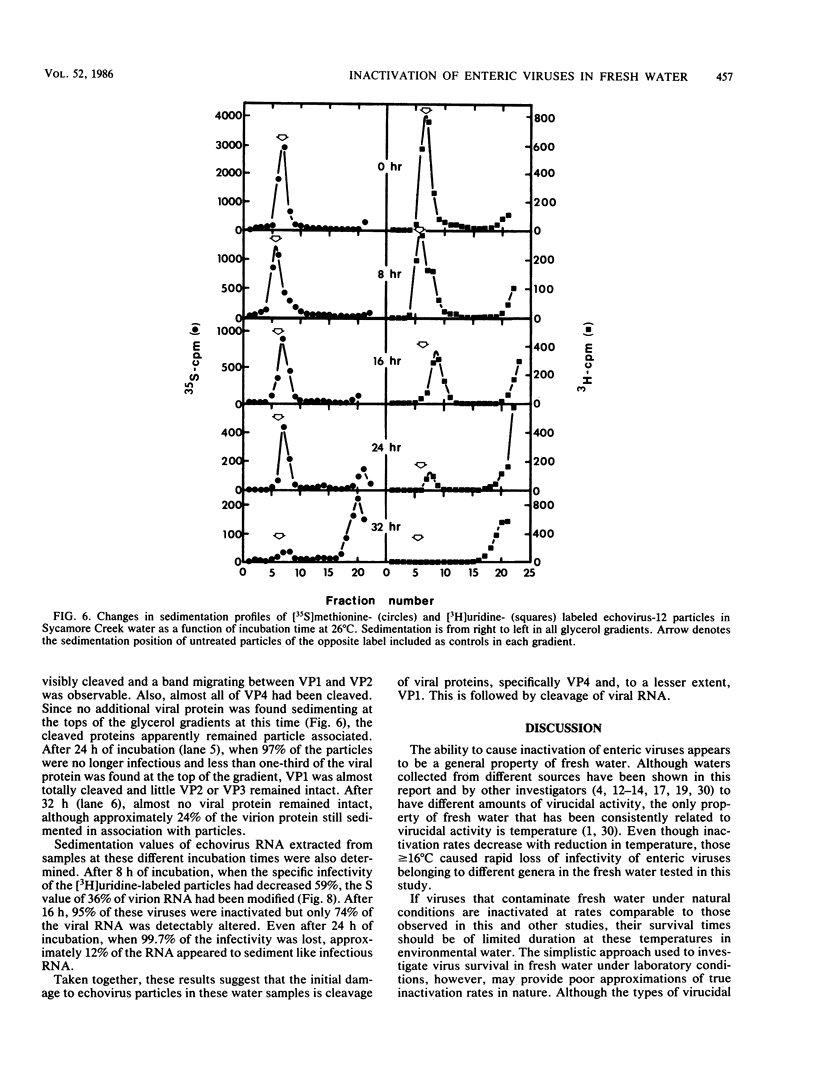
Abstract
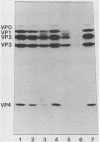
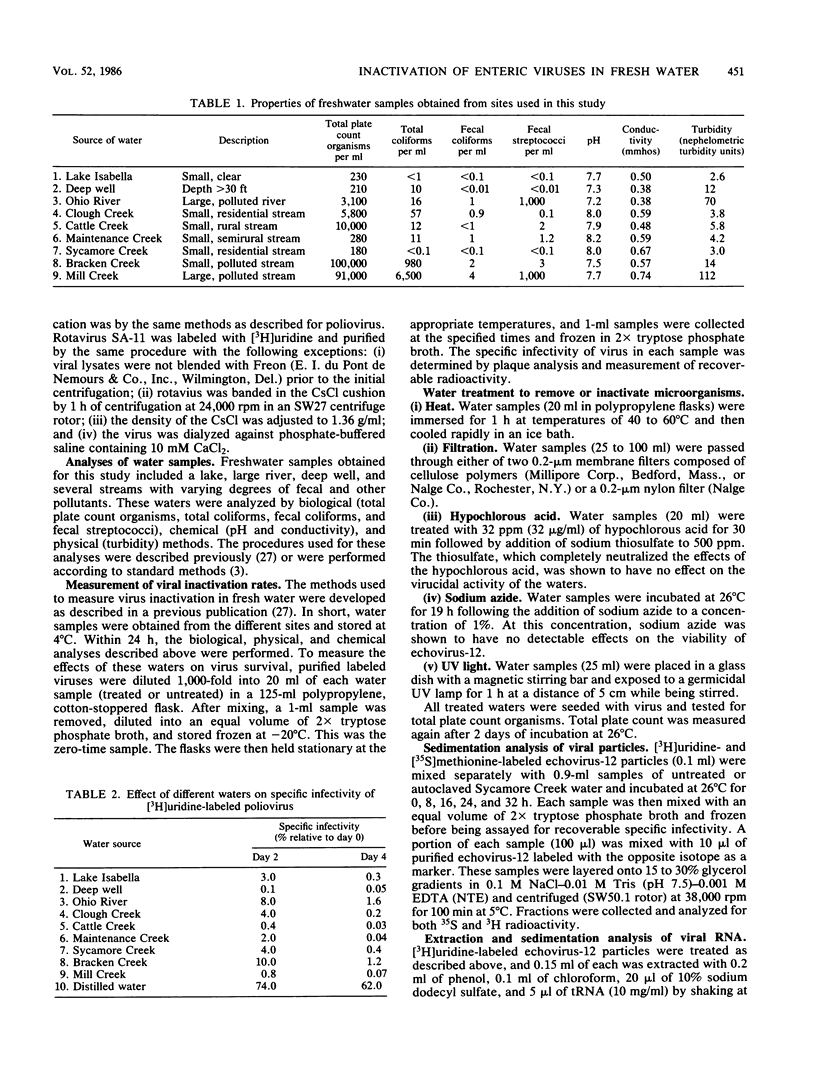
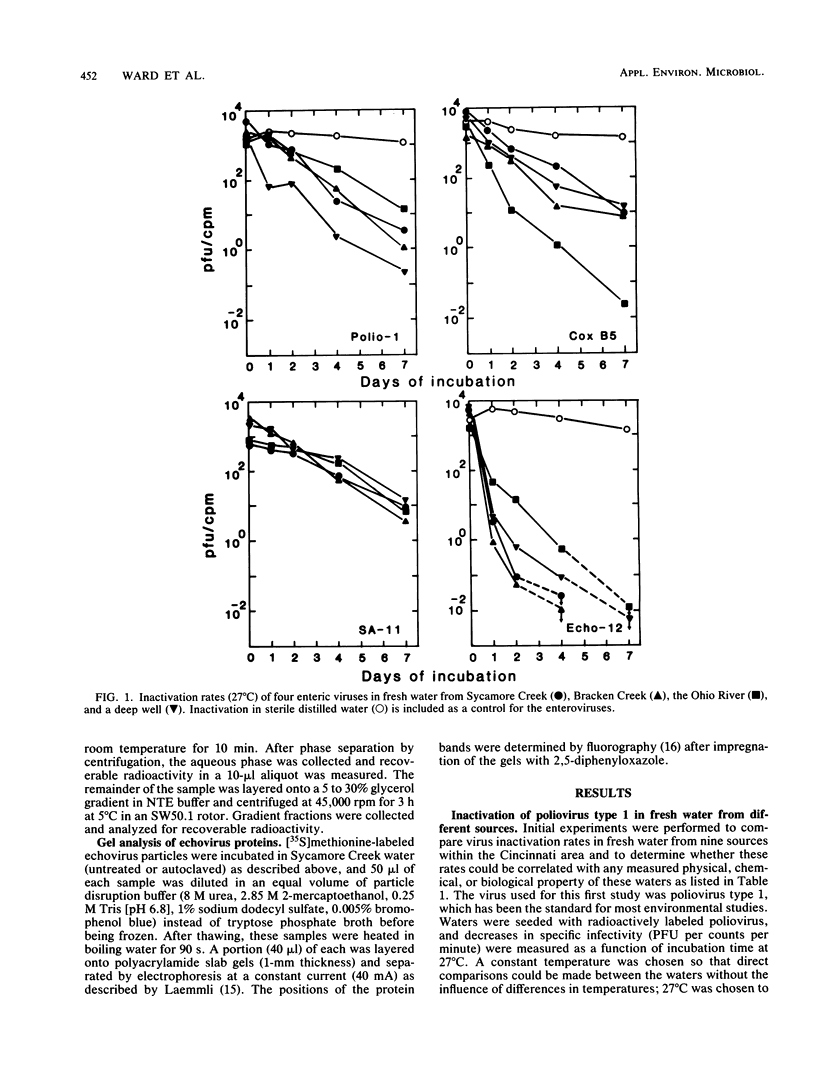
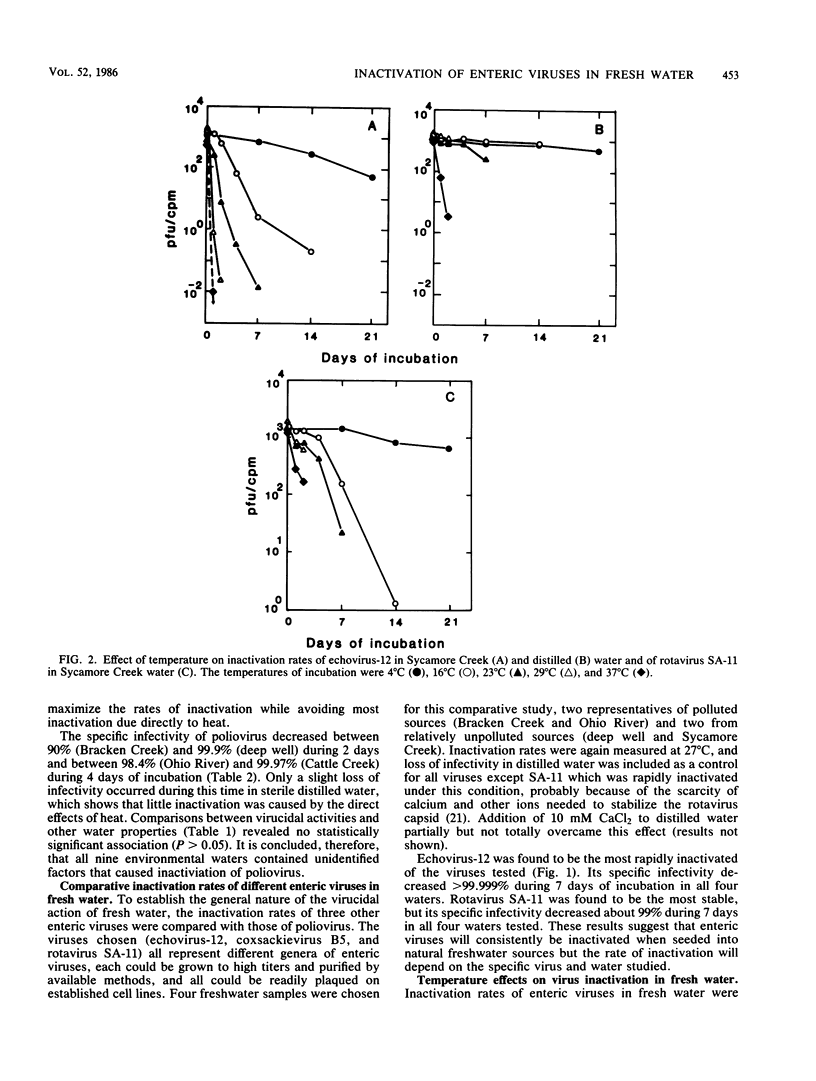
Fresh water obtained from nine sources was shown to cause inactivation of poliovirus. Further testing with four of these water samples showed that enteric viruses from different genera were consistently inactivated in these freshwater samples. Studies on the cause of inactivation were conducted with echovirus type 12 as the model virus. The results revealed that the virucidal agents in the waters tested could not be separated from microorganisms. Any treatment that removed or inactivated microorganisms caused loss of virucidal activity. Microbial growth in a sterilized creek water seeded with a small amount of stream water resulted in concomitant production of virucidal activity. When individual bacterial isolates obtained from a stream were grown in this sterilized creek water, most (22 of 27) produced a large amount of virucidal activity, although the amount varied from one isolate to the next. Active and inactive isolates were represented by both gram-positive and gram-negative organisms. Examination of echoviruses inactivated in stream water revealed that loss of infectivity first correlated with a slight decrease in the sedimentation coefficient of virus particles. The cause appeared to be cleavage of viral proteins, most notably, VP-4 and, to a lesser extent, VP-1. Viral RNA associated with particles was also cleaved but the rate was slower than loss of infectivity. These results suggest that proteolytic bacterial enzymes inactivate echovirus particles in fresh water by cleavage of viral proteins, thus exposing the viral RNA to nuclease digestion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breindl M. The structure of heated poliovirus particles. J Gen Virol. 1971 Jun;11(3):147–156. doi: 10.1099/0022-1317-11-3-147. [DOI] [PubMed] [Google Scholar]
- Fujioka R. S., Loh P. C., Lau L. S. Survival of human enteroviruses in the Hawaiian ocean environment: evidence for virus-inactivating microorganisms. Appl Environ Microbiol. 1980 Jun;39(6):1105–1110. doi: 10.1128/aem.39.6.1105-1110.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Schaiberger G. E. Effect of particulates on virus survival in seawater. J Water Pollut Control Fed. 1975 Jan;47(1):93–103. [PubMed] [Google Scholar]
- Herrmann J. E., Cliver D. O. Degradation of coxsackievirus type A9 by proteolytic enzymes. Infect Immun. 1973 Apr;7(4):513–517. doi: 10.1128/iai.7.4.513-517.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Kostenbader K. D., Jr, CLIVER D. O. Persistence of enteroviruses in lake water. Appl Microbiol. 1974 Nov;28(5):895–896. doi: 10.1128/am.28.5.895-896.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst C. J., Gerba C. P. Stability of simian rotavirus in fresh and estuarine water. Appl Environ Microbiol. 1980 Jan;39(1):1–5. doi: 10.1128/aem.39.1.1-5.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mahnel H., Ottis K., Herlyn M. Stabilität von neun Virusarten unterschiedlicher Genera in Trinkund Oberflächenwasser. Zentralbl Bakteriol Orig B. 1977 Jan;164(1-2):64–84. [PubMed] [Google Scholar]
- O'Brien R. T., Newman J. S. Inactivation of polioviruses and coxsackieviruses in surface water. Appl Environ Microbiol. 1977 Feb;33(2):334–340. doi: 10.1128/aem.33.2.334-340.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley J. A., Beards G. M., Thouless M. E., Flewett T. H. The influence of divalent cations on the stability of human rotavirus. Arch Virol. 1981;67(1):1–9. doi: 10.1007/BF01314596. [DOI] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Hetrick F. M. Antiviral activity of antibiotic-producing marine bacteria. Can J Microbiol. 1982 Feb;28(2):231–238. doi: 10.1139/m82-031. [DOI] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Hetrick F. M. Mechanism of poliovirus inactivation by cell-free filtrates of marine bacteria. Can J Microbiol. 1983 Nov;29(11):1481–1486. doi: 10.1139/m83-228. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. Inactivation of poliovirus in digested sludge. Appl Environ Microbiol. 1976 Jun;31(6):921–930. doi: 10.1128/aem.31.6.921-930.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Pierce M. J. Efficiency of human rotavirus propagation in cell culture. J Clin Microbiol. 1984 Jun;19(6):748–753. doi: 10.1128/jcm.19.6.748-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Winston P. E. Development of methods to measure virus inactivation in fresh waters. Appl Environ Microbiol. 1985 Nov;50(5):1144–1148. doi: 10.1128/aem.50.5.1144-1148.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Mountain C. W. Demonstration of solids-associated virus in wastewater and sludge. Appl Environ Microbiol. 1976 Mar;31(3):354–358. doi: 10.1128/aem.31.3.354-358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. V., Gerba C. P., Kelley L. M. Virus persistence in groundwater. Appl Environ Microbiol. 1985 Apr;49(4):778–781. doi: 10.1128/aem.49.4.778-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]