Abstract
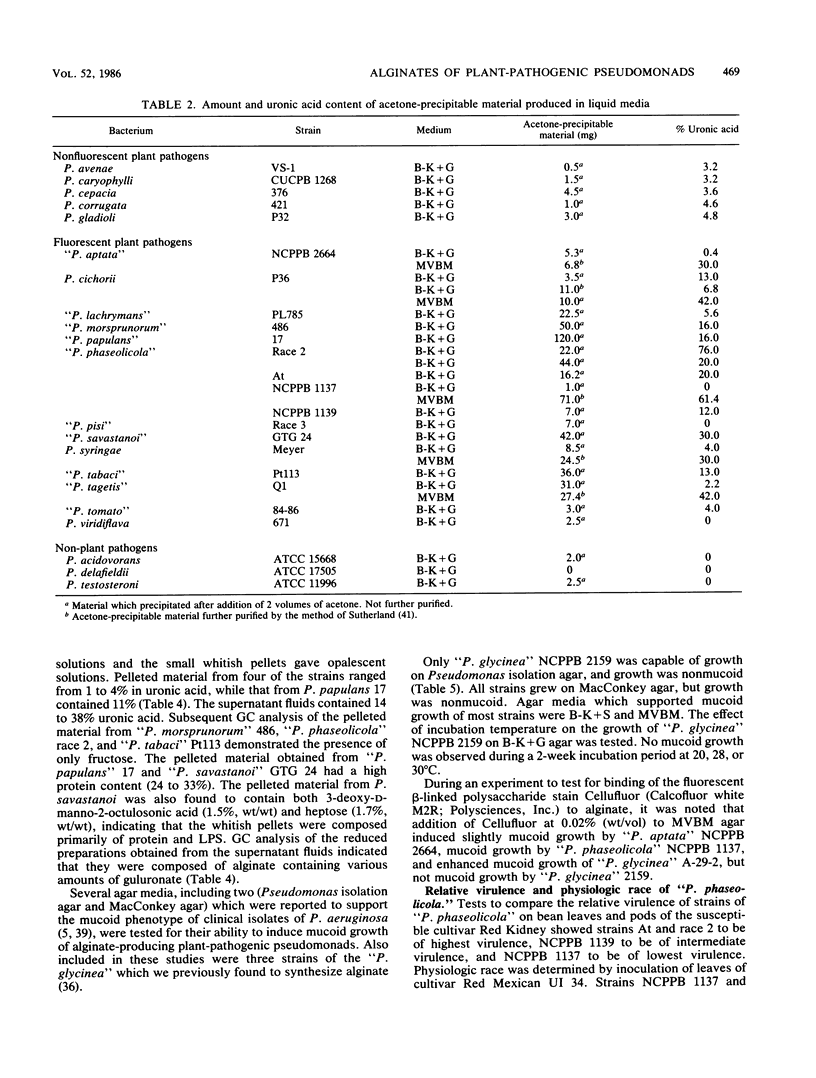
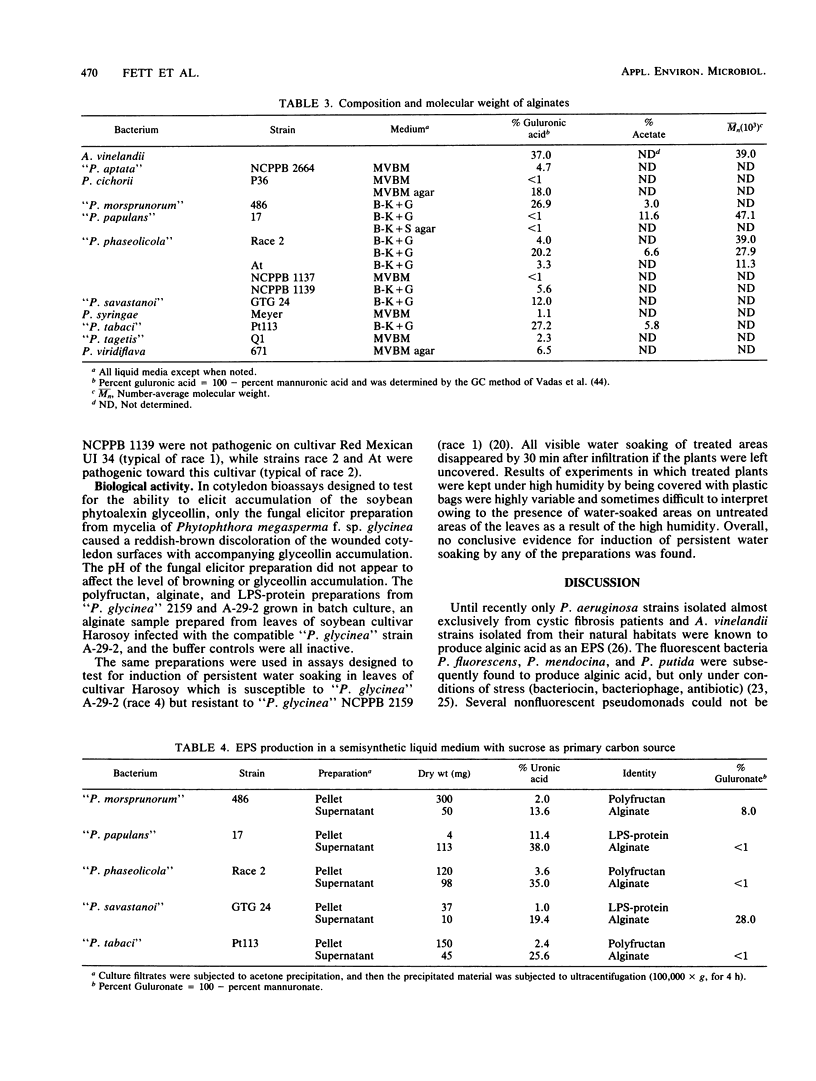
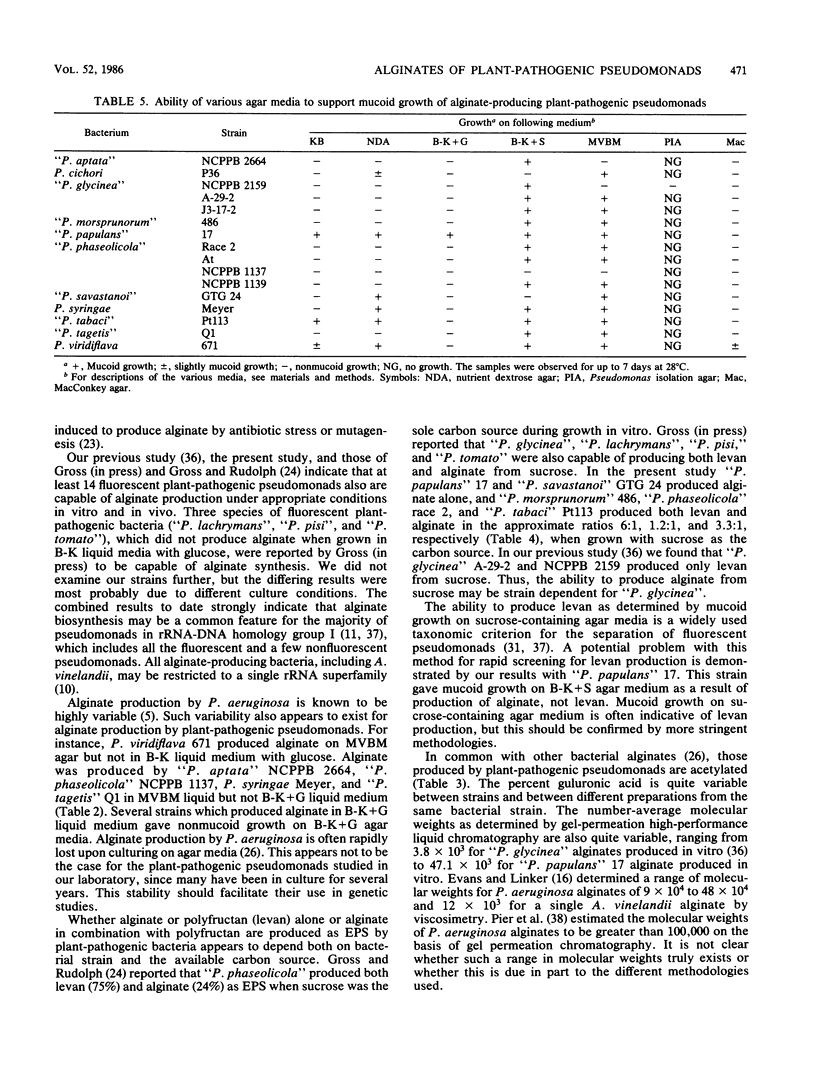
Eighteen plant-pathogenic and three non-plant-pathogenic pseudomonads were tested for the ability to produce alginic acid as an exopolysaccharide in vitro. Alginate production was demonstrated for 10 of 13 fluorescent plant-pathogenic pseudomonads tested with glucose or gluconate as the carbon source, but not for all 5 nonfluorescent plant pathogens and all 3 non-plant pathogens tested. With sucrose as the carbon source, some strains produced alginate while others produced both polyfructan (levan) and alginate. Alginates ranged from <1 to 28% guluronic acid, were acetylated, and had number-average molecular weights of 11.3 × 103 to 47.1 × 103. Polyfructans and alginates were not elicitors of the soybean phytoalexin glyceollin when applied to wounded cotyledon surfaces and did not induce prolonged water soaking of soybean leaf tissues. All or most pseudomonads in rRNA-DNA homology group I may be capable of synthesizing alginate as an exopolysaccharide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bucke C. Polyacrylamide gel electrophoresis of alginic acid. J Chromatogr. 1974 Feb 13;89(1):99–102. doi: 10.1016/s0021-9673(01)84167-4. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L. Influence of nutrient media on the characteristics of the exopolysaccharide produced by three mucoid Pseudomonas aeruginosa strains. Microbios. 1984;41(163):49–63. [PubMed] [Google Scholar]
- Chan R., Lam J. S., Lam K., Costerton J. W. Influence of culture conditions on expression of the mucoid mode of growth of Pseudomonas aeruginosa. J Clin Microbiol. 1984 Jan;19(1):8–16. doi: 10.1128/jcm.19.1.8-16.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E. A., Preston J. F. Enzyme formation and polysaccharide synthesis by bacteria. Biochem J. 1935 Oct;29(10):2267–2277. doi: 10.1042/bj0292267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drigues P., Demery-Lafforgue D., Trigalet A., Dupin P., Samain D., Asselineau J. Comparative studies of lipopolysaccharide and exopolysaccharide from a virulent strain of Pseudomonas solanacearum and from three avirulent mutants. J Bacteriol. 1985 May;162(2):504–509. doi: 10.1128/jb.162.2.504-509.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod R. P., Braun A. C. Pseudomonas aeruginosa: Its Rôle as a Plant Pathogen. J Bacteriol. 1942 Dec;44(6):633–645. doi: 10.1128/jb.44.6.633-645.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A., Jarman T. R. Isolation of alginate-producing mutants of Pseudomonas fluorescens, Pseudomonas putida and Pseudomonas mendocina. J Gen Microbiol. 1981 Jul;125(1):217–220. doi: 10.1099/00221287-125-1-217. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Lelliott R. A., Billing E., Hayward A. C. A determinative scheme for the fluorescent plant pathogenic pseudomonads. J Appl Bacteriol. 1966 Dec;29(3):470–489. doi: 10.1111/j.1365-2672.1966.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Osman S. F., Fett W. F., Fishman M. L. Exopolysaccharides of the phytopathogen Pseudomonas syringae pv. glycinea. J Bacteriol. 1986 Apr;166(1):66–71. doi: 10.1128/jb.166.1.66-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Matthews W. J., Jr, Eardley D. D. Immunochemical characterization of the mucoid exopolysaccharide of Pseudomonas aeruginosa. J Infect Dis. 1983 Mar;147(3):494–503. doi: 10.1093/infdis/147.3.494. [DOI] [PubMed] [Google Scholar]
- Pugashetti B. K., Metzger H. M., Jr, Vadas L., Feingold D. S. Phenotypic differences among clinically isolated mucoid Pseudomonas aeruginosa strains. J Clin Microbiol. 1982 Oct;16(4):686–691. doi: 10.1128/jcm.16.4.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P. Host defenses in patients with cystic fibrosis: modulation by Pseudomonas aeruginosa. Surv Synth Pathol Res. 1985;4(1):14–33. doi: 10.1159/000156962. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vadas L., Prihar H. S., Pugashetti B. K., Feingold D. S. A gas chromatographic method for the quantitative determination of hexuronic acids in alginic acid. Anal Biochem. 1981 Jul 1;114(2):294–298. doi: 10.1016/0003-2697(81)90484-x. [DOI] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]


