Abstract
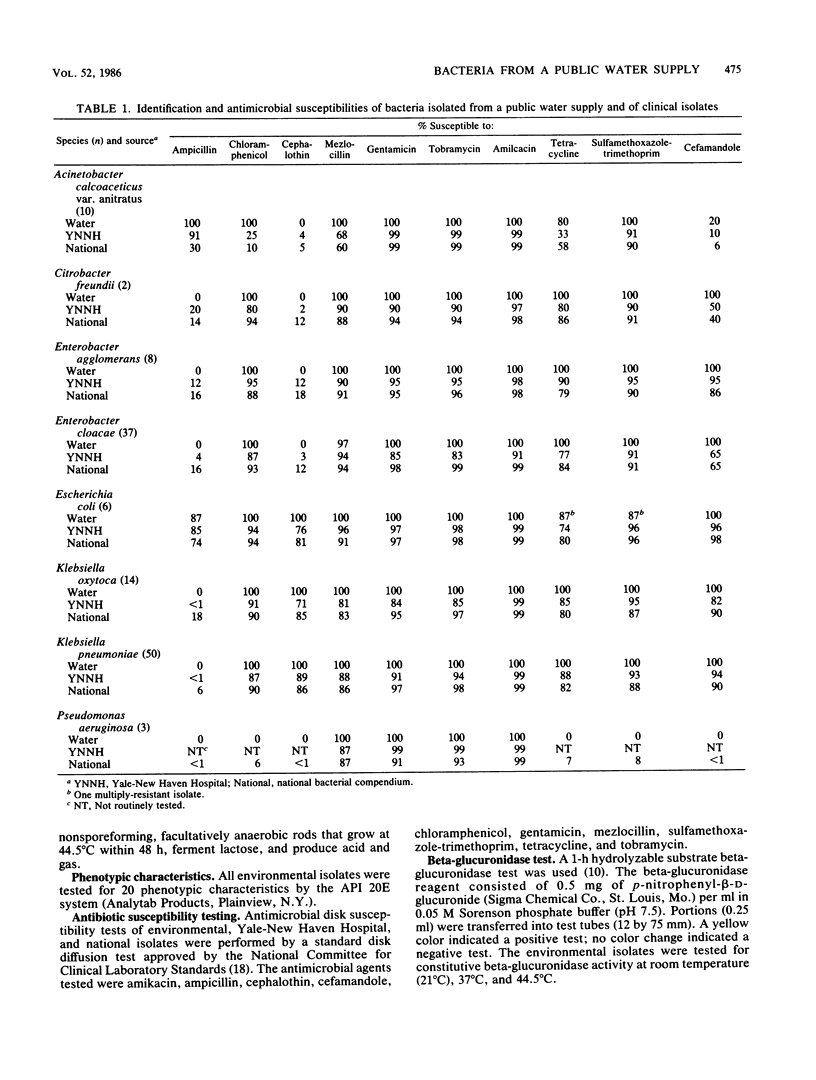
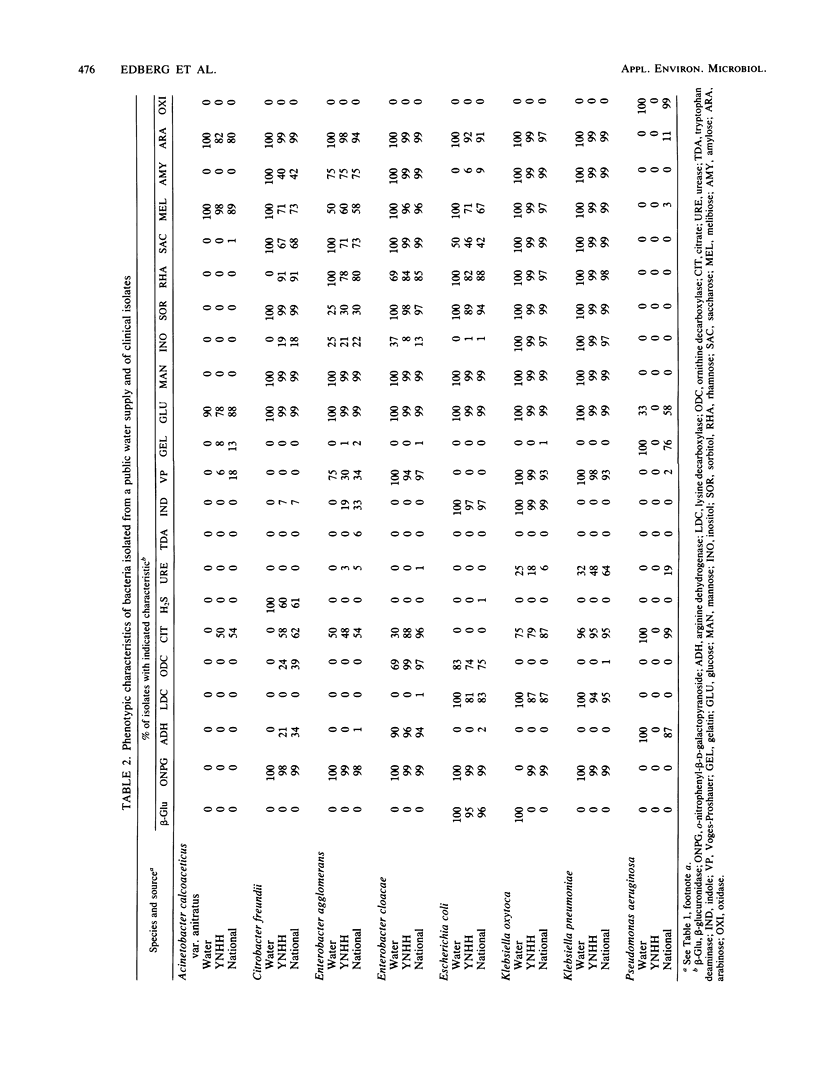
During the summer and fall of 1984, elevated total coliform counts were observed in the distribution system of a public water supply serving 350,000 people in south central Connecticut. As part of an investigation of possible health risks associated with the presence of bacteria in the water supply, bacterial isolates from the distribution system were compared with bacterial isolates of the same species obtained from a large regional teaching hospital and from a national compendium of clinical isolates. Characteristics analyzed included phenotypic metabolic activity, antimicrobial susceptibilities to clinically utilized antibiotics, temperature tolerance at 44.5 degrees C, and beta-glucuronidase activity in single-test form and on a selective medium. Environmental isolates lacked known plasmid-mediated characteristics, with the exception of one Escherichia coli isolate which showed some antibiotic resistance. Klebsiella pneumoniae, Klebsiella oxytoca, Enterobacter cloacae, and Enterobacter agglomerans from all sources were temperature tolerant and yielded positive fecal coliform tests. Only E. coli showed beta-glucuronidase activity (both in a single biochemical test and on a selective medium). No single characteristic analyzed was sufficient to establish an organism as either environmental or clinical in origin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. L., Calomiris J. J., Seidler R. J. Selection of antibiotic-resistant standard plate count bacteria during water treatment. Appl Environ Microbiol. 1982 Aug;44(2):308–316. doi: 10.1128/aem.44.2.308-316.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson B. A., Lorian V. Antimicrobial agent susceptibility patterns of bacteria in hospitals from 1971 to 1982. J Clin Microbiol. 1984 Oct;20(4):791–796. doi: 10.1128/jcm.20.4.791-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley S. T., Seidler R. J. Significance of fecal coliform-positive Klebsiella. Appl Environ Microbiol. 1977 May;33(5):1141–1148. doi: 10.1128/aem.33.5.1141-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplenas N. R., Kanarek M. S. Thermotolerant non-fecal source Klebsiella pneumoniae: validity of the fecal coliform test in recreational waters. Am J Public Health. 1984 Nov;74(11):1273–1275. doi: 10.2105/ajph.74.11.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian R. R., Pipes W. O. Frequency distribution of coliforms in water distribution systems. Appl Environ Microbiol. 1983 Feb;45(2):603–609. doi: 10.1128/aem.45.2.603-609.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Trepeta R. W. Rapid and economical identification and antimicrobial susceptibility test methodology for urinary tract pathogens. J Clin Microbiol. 1983 Dec;18(6):1287–1291. doi: 10.1128/jcm.18.6.1287-1291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson A. S., Cooke E. M., Wilcock A. P., Shinebaum R. A comparison of the properties of Klebsiella strains isolated from different sources. J Med Microbiol. 1980 Nov;13(4):541–550. doi: 10.1099/00222615-13-4-541. [DOI] [PubMed] [Google Scholar]
- Feng P. C., Hartman P. A. Fluorogenic assays for immediate confirmation of Escherichia coli. Appl Environ Microbiol. 1982 Jun;43(6):1320–1329. doi: 10.1128/aem.43.6.1320-1329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Bülow P. Rapid diagnosis of Enterobacteriaceae. I. Detection of bacterial glycosidases. Acta Pathol Microbiol Scand B. 1976 Oct;84B(5):245–251. doi: 10.1111/j.1699-0463.1976.tb01933.x. [DOI] [PubMed] [Google Scholar]
- Naemura L. G., Seidler R. J. Significance of low-temperature growth associated with the fecal coliform response, indole production, and pectin liquefaction in Klebsiella. Appl Environ Microbiol. 1978 Feb;35(2):392–396. doi: 10.1128/aem.35.2.392-396.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison B. J. Evaluation of a fluorogenic assay for detection of Escherichia coli in foods. Appl Environ Microbiol. 1984 Aug;48(2):285–288. doi: 10.1128/aem.48.2.285-288.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot H. W., Jr, Yamamoto D. K., Smith M. W., Seidler R. J. Antibiotic resistance and its transfer among clinical and nonclinical Klebsiella strains in botanical environments. Appl Environ Microbiol. 1980 Jan;39(1):97–104. doi: 10.1128/aem.39.1.97-104.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepeta R. W., Edberg S. C. Methylumbelliferyl-beta-D-glucuronide-based medium for rapid isolation and identification of Escherichia coli. J Clin Microbiol. 1984 Feb;19(2):172–174. doi: 10.1128/jcm.19.2.172-174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



