Abstract
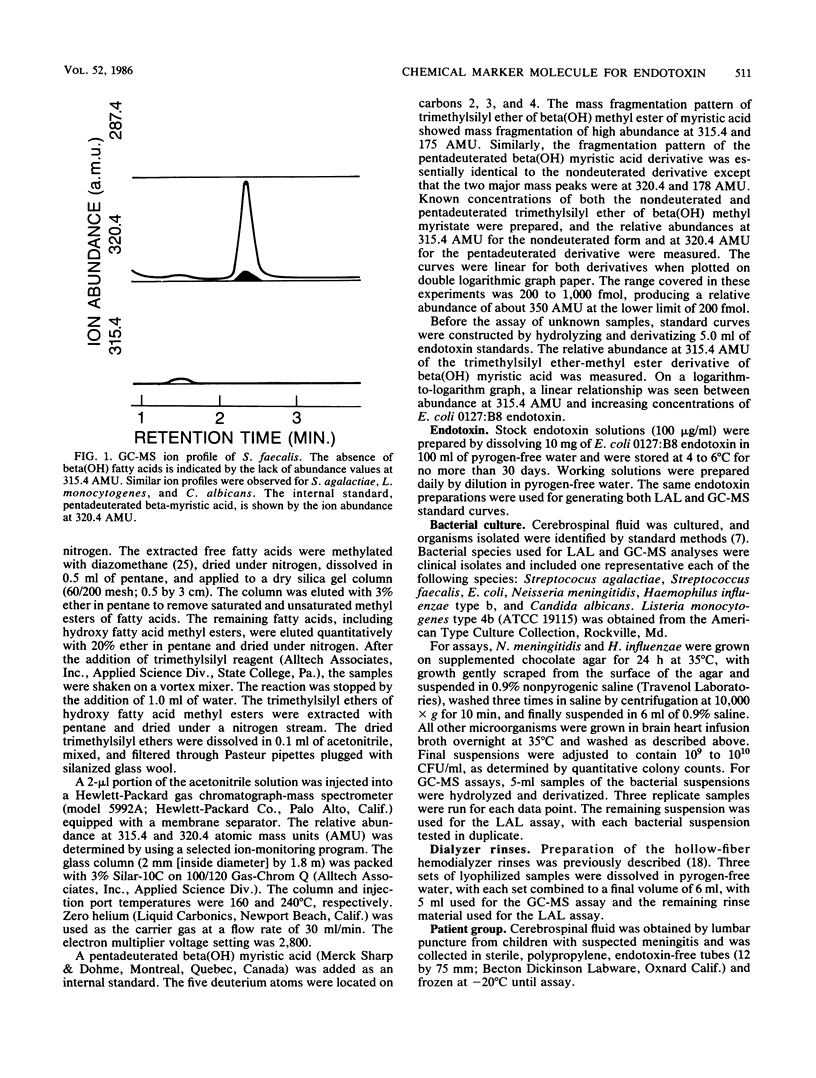
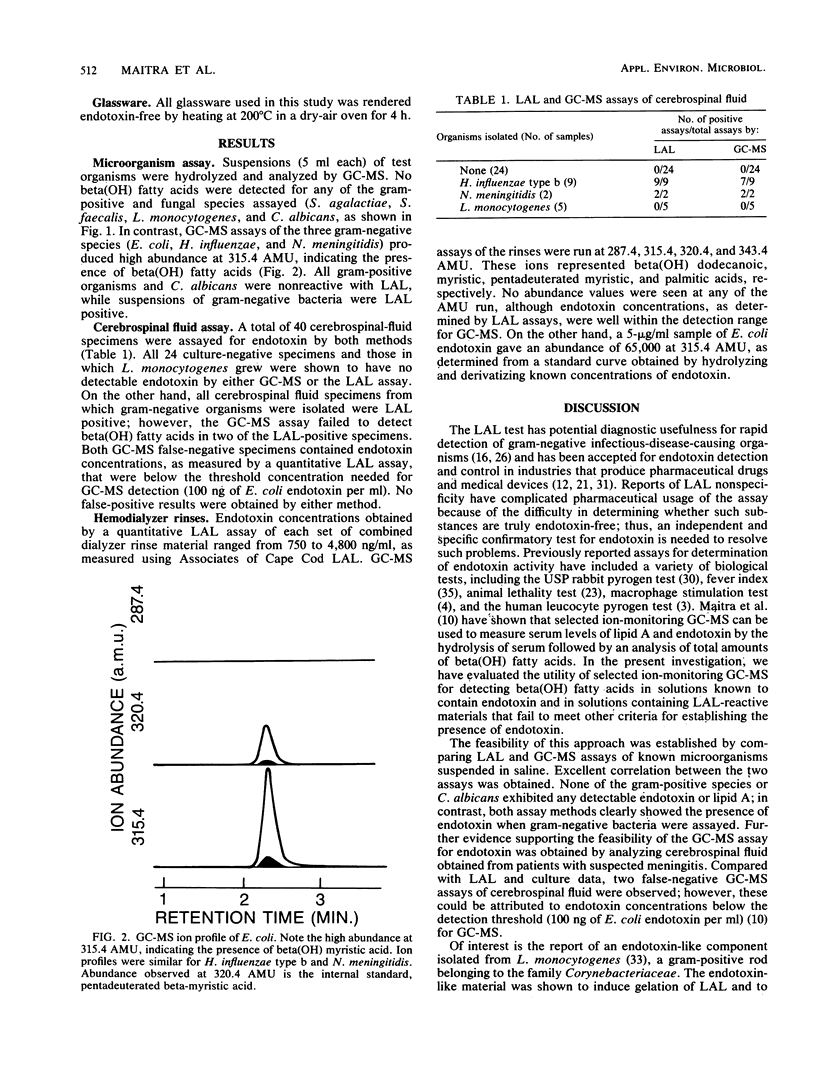
Selected ion-monitoring gas chromatography-mass spectrometry was used for detection of beta-hydroxy fatty acids as an independent assay for the presence or absence of endotoxin in materials claimed to induce nonspecific activation of Limulus amoebocyte lysate. To this end, suspensions of gram-negative and -positive bacteria, one fungal species, cerebrospinal fluid, and hollow-fiber hemodialyzer rinses were assayed for endotoxin by gas chromatography-mass spectrometry and the Limulus amoebocyte lysate assay. Good qualitative agreement was shown for both methods when suspensions of test organisms were assayed. Two false-negative results were obtained by gas chromatography-mass spectrometry assays of cerebrospinal fluid and were shown to be a result of insufficient endotoxin in the cerebrospinal fluid specimens for detection by gas chromatography-mass spectrometry. Hemodialyzer rinses were Limulus assay positive; however, no beta-hydroxy fatty acids were detected by gas chromatography-mass spectrometry. These data were compared with data obtained from USP rabbit pyrogen tests of the rinse materials (nonpyrogenic) and chemical characterization of the Limulus assay-reactive rinses, which showed the rinses to be cellulosic in nature. It is suggested that beta-hydroxy fatty acids, as assayed by selected ion-monitoring gas chromatography-mass spectrometry, be used as chemical marker molecules for the presence or absence of endotoxin in materials reported to cause nonspecific activation of Limulus amoebocyte lysate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunson K. W., Watson D. W. Limulus amebocyte lysate reaction with streptococcal pyrogenic exotoxin. Infect Immun. 1976 Nov;14(5):1256–1258. doi: 10.1128/iai.14.5.1256-1258.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L. A., Petersen N. J. LAL-reactive material associated with hemodialysis membranes. Prog Clin Biol Res. 1982;93:217–230. [PubMed] [Google Scholar]
- Dinarello C. A., O'Connor J. V., LoPreste G., Swift R. L. Human leukocytic pyrogen test for detection of pyrogenic material in growth hormone produced by recombinant Escherichia coli. J Clin Microbiol. 1984 Sep;20(3):323–329. doi: 10.1128/jcm.20.3.323-329.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe W. F., Yang S. T., Morrison D. C., Betz S. J., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. II. Evidence for differentiation signals delivered by lipid A and by a protein rich fraction of lipopolysaccharides. J Exp Med. 1978 Aug 1;148(2):557–568. doi: 10.1084/jem.148.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Utter A. E. Positive Limulus amoebocyte lysate reactions with polyriboinosinic acid x polyribocytidylic acid. J Clin Microbiol. 1980 Oct;12(4):502–505. doi: 10.1128/jcm.12.4.502-505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Wolff S. M. Nonspecificity of the limulus amebocyte lysate test: positive reactions with polynucleotides and proteins. J Infect Dis. 1973 Sep;128(3):349–352. doi: 10.1093/infdis/128.3.349. [DOI] [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. THE ROLE OF ENDOTOXIN IN THE EXTRACELLULAR COAGULATION OF LIMULUS BLOOD. Bull Johns Hopkins Hosp. 1964 Sep;115:265–274. [PubMed] [Google Scholar]
- Maitra S. K., Schotz M. C., Yoshikawa T. T., Guze L. B. Determination of lipid A and endotoxin in serum by mass spectroscopy. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3993–3997. doi: 10.1073/pnas.75.8.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T., Nagase T., Matsumoto, Suzuki S., Suzuki M. Gelatin of Limulus amoebocyte lysate by simple polysaccharides. Microbiol Immunol. 1982;26(5):403–409. doi: 10.1111/j.1348-0421.1982.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Munson T. E. FDA guideline for validation of the LAL test as an end-product endotoxin test for human and biological drugs. Prog Clin Biol Res. 1982;93:25–32. [PubMed] [Google Scholar]
- Nachum R., Berzofsky R. N. Chromogenic Limulus amoebocyte lysate assay for rapid detection of gram-negative bacteriuria. J Clin Microbiol. 1985 May;21(5):759–763. doi: 10.1128/jcm.21.5.759-763.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachum R., Christiansen R. O. Rapid presumptive diagnosis of gonococcal urethritis in males: evaluation of a prototype limulus lysate test kit. Med Lab Sci. 1982 Jan;39(1):83–85. [PubMed] [Google Scholar]
- Nachum R., Lipsey A., Siegel S. E. Rapid detection of gram-negative bacterial meningitis by the limulus lysate test. N Engl J Med. 1973 Nov 1;289(18):931–934. doi: 10.1056/NEJM197311012891801. [DOI] [PubMed] [Google Scholar]
- Nachum R., Shanbrom E. Rapid detection of Gram-negative bacteriuria by Limulus amoebocyte lysate assay. J Clin Microbiol. 1981 Jan;13(1):158–162. doi: 10.1128/jcm.13.1.158-162.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson F. C., 3rd, Weary M. E., Bohon J., Dabbah R. Relative potency of "environmental" endotoxin as measured by the Limulus amebocyte lysate test and the USP rabbit pyrogen test. Prog Clin Biol Res. 1982;93:65–77. [PubMed] [Google Scholar]
- Pearson F. C., 3rd, Weary M. E., Dabbah R. A corporate approach to in-process and end-product testing with the LAL assay for endotoxin. Prog Clin Biol Res. 1982;93:231–246. [PubMed] [Google Scholar]
- Pearson F. C., Bohon J., Lee W., Bruszer G., Sagona M., Jakubowski G., Dawe R., Morrison D., Dinarello C. Characterization of Limulus amoebocyte lysate-reactive material from hollow-fiber dialyzers. Appl Environ Microbiol. 1984 Dec;48(6):1189–1196. doi: 10.1128/aem.48.6.1189-1196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson F. C., Weary M., Bohon J. Detection of Limulus amebocyte lysate reactive material in capillary flow hemodialyzers. Prog Clin Biol Res. 1982;93:247–260. [PubMed] [Google Scholar]
- Petersen N. J., Carson L. A., Favero M. S. Bacterial endotoxin in new and reused hemodialyzers: a potential cause of endotoxemia. Trans Am Soc Artif Intern Organs. 1981;27:155–160. [PubMed] [Google Scholar]
- Pieroni R. E., Broderick E. J., Bundeally A., Levine L. A simple method for the quantitation of submicrogram amounts of bacterial endotoxin. Proc Soc Exp Biol Med. 1970 Mar;133(3):790–794. doi: 10.3181/00379727-133-34565. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Spagna V. A., Prior R. B., Perkins R. L. Rapid presumptive diagnosis of gonococcal cervicitis by the limulus lysate assay. Am J Obstet Gynecol. 1980 Jul 1;137(5):595–599. doi: 10.1016/0002-9378(80)90702-4. [DOI] [PubMed] [Google Scholar]
- Spagna V. A., Prior R. B., Perkins R. L. Rapid presumptive diagnosis of gonococcal urethritis in men by the limulus lysate test. Br J Vener Dis. 1979 Jun;55(3):179–182. doi: 10.1136/sti.55.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Mikami T., Matsumoto T., Suzuki S. Gelation of Limulus lysate by synthetic dextran derivatives. Microbiol Immunol. 1977;21(8):419–425. doi: 10.1111/j.1348-0421.1977.tb00307.x. [DOI] [PubMed] [Google Scholar]
- WOLFF S. M., MULHOLLAND J. H., WARD S. B. QUANTITATIVE ASPECTS OF THE PYROGENIC RESPONSE OF RABBITS TO ENDOTOXIN. J Lab Clin Med. 1965 Feb;65:268–275. [PubMed] [Google Scholar]
- Weary M., Baker B. Utilization of the limulus amebocyte lysate test for pyrogen testing large volume parenterals, administration sets, and medical devices. Bull Parenter Drug Assoc. 1977 May-Jun;31(3):127–133. [PubMed] [Google Scholar]
- Westphal O. Bacterial endotoxins. The second Carl Prausnitz Memorial Lecture. Int Arch Allergy Appl Immunol. 1975;49(1-2):1–43. [PubMed] [Google Scholar]
- Wexler H., Oppenheim J. D. Isolation, characterization, and biological properties of an endotoxin-like material from the gram-positive organism Listeria monocytogenes. Infect Immun. 1979 Mar;23(3):845–857. doi: 10.1128/iai.23.3.845-857.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildfeuer A., Heymer B., Schleifer K. H., Haferkamp O. Investigations on the specificity of the Limulus test for the detection of endotoxin. Appl Microbiol. 1974 Nov;28(5):867–871. doi: 10.1128/am.28.5.867-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]


