Abstract
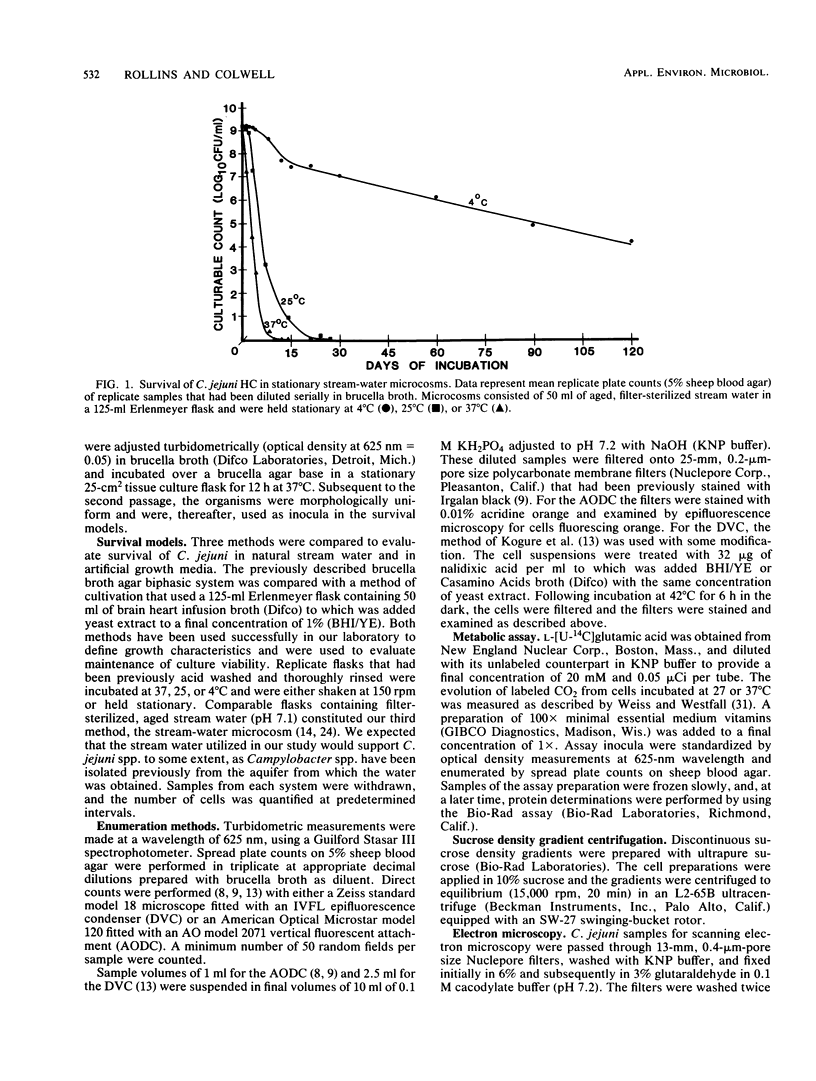
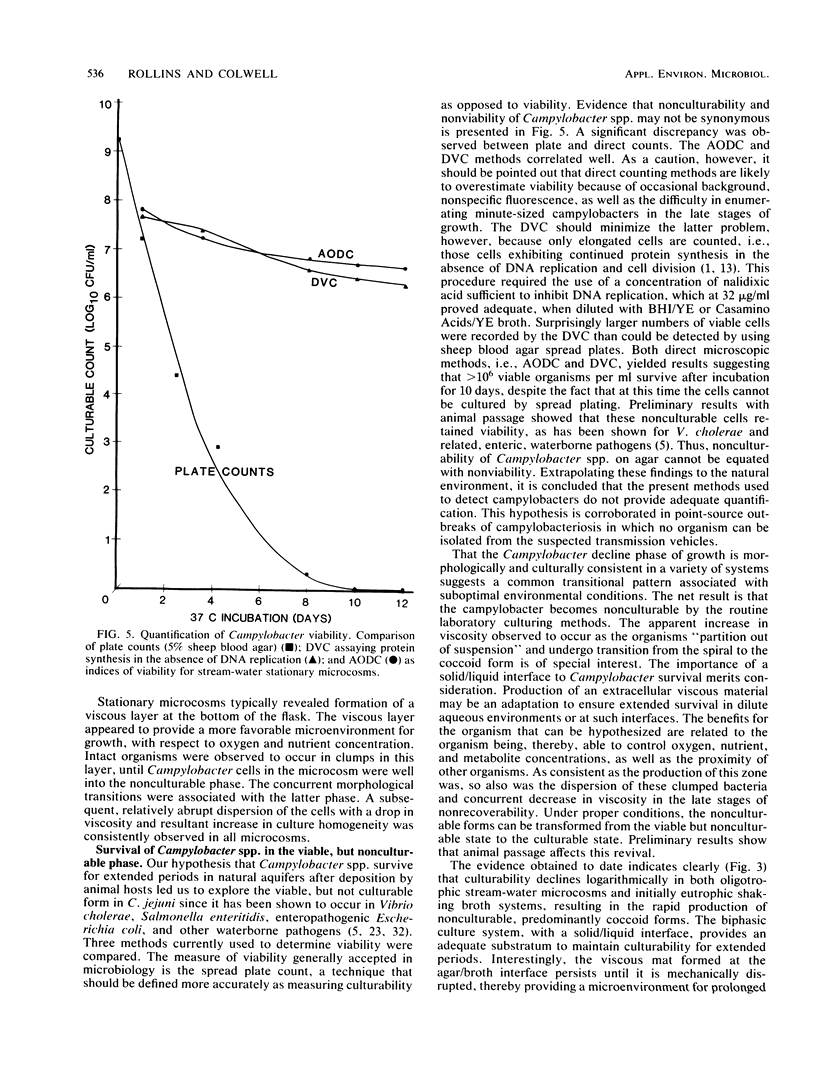
Conditions influencing the survival of Campylobacter jejuni in the natural aquatic environment have been determined. Release of Campylobacter spp. into natural waters by animal hosts is postulated to play a key role in the maintenance of viability and transmission of the organism in the environment. Laboratory flask microcosms containing filter-sterilized stream water were used to test C. jejuni for the ability to remain viable in simulated natural systems. The microcosms were compared with the biphasic and shaking broth procedures used routinely for growth of Campylobacter spp. in the research laboratory. The stream-water microcosms were analyzed to determine effects of temperature and aeration on the survival of a well-characterized C. jejuni strain isolated originally from a human campylobacteriosis patient. Morphological characteristics were evaluated by phase-contrast microscopy and scanning or transmission electron microscopy. Survival curves were quantified on the basis of plate counts, epifluorescent microscopy, optical density measurements, and direct viable counts associated with protein synthesis in the absence of DNA replication. A significant difference was observed between results of direct enumeration, i.e., direct viable counts or acridine orange direct counts, and those from spread plate cultures. In all cases, increasing temperature of cultivation resulted in decreased recoverability on laboratory media, due possibly to an increased metabolic rate, as analyzed by CO2 evolution in the presence of radiolabeled glutamate. Stream water held at low temperature (4 degrees C) sustained significant numbers of campylobacters for greater than 4 months. Microcosms, aerated with shaking, exhibited logarithmic decline in recoverable C. jejuni, while stationary systems underwent a more moderate rate of decrease to the nonculturable state.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. M., Singleton F. L., Hood M. A. Effects of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol. 1983 Oct;46(4):930–940. doi: 10.1128/aem.46.4.930-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hardesty H. L., Powers B., Wang W. L. Survival of Campylobacter fetus subsp. jejuni in biological milieus. J Clin Microbiol. 1980 Apr;11(4):309–313. doi: 10.1128/jcm.11.4.309-313.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Taylor D. N., Feldman R. A. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- CHATTERJEE B. R., WILLIAMS R. P. Cytological changes in aging bacterial cultures. J Bacteriol. 1962 Aug;84:340–344. doi: 10.1128/jb.84.2.340-344.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felter R. A., Colwell R. R., Chapman G. B. Morphology and round body fermation in Vibrio marinus. J Bacteriol. 1969 Jul;99(1):326–335. doi: 10.1128/jb.99.1.326-335.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco D. E., Mah R. A., Rabin A. C. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Trans Am Microsc Soc. 1973 Jul;92(3):416–421. [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Saito K., Maruyama T., Sakai S., Ohashi M., Oka A. An outbreak of acute enteritis due to Campylobacter fetus subspecies jejuni at a nursery school of Tokyo. Microbiol Immunol. 1980;24(5):371–379. doi: 10.1111/j.1348-0421.1980.tb02841.x. [DOI] [PubMed] [Google Scholar]
- Karmali M. A., Fleming P. C. Campylobacter enteritis. Can Med Assoc J. 1979 Jun 23;120(12):1525–1532. [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Kosinski R. J., Singleton F. L., Foster B. G. Sampling culturable heterotrophs from microcosms: a statistical analysis. Appl Environ Microbiol. 1979 Nov;38(5):906–910. doi: 10.1128/aem.38.5.906-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg N. R. Biology of the chemoheterotrophic spirilla. Bacteriol Rev. 1976 Mar;40(1):55–115. doi: 10.1128/br.40.1.55-115.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson J. J., Keswick B. H., DuPont H. L. Evaluation of filters for recovery of Campylobacter jejuni from water. Appl Environ Microbiol. 1983 Nov;46(5):985–987. doi: 10.1128/aem.46.5.985-987.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzing L. O. Waterborne outbreaks of campylobacter enteritis in central Sweden. Lancet. 1981 Aug 15;2(8242):352–354. doi: 10.1016/s0140-6736(81)90658-9. [DOI] [PubMed] [Google Scholar]
- NG L. K., Sherburne R., Taylor D. E., Stiles M. E. Morphological forms and viability of Campylobacter species studied by electron microscopy. J Bacteriol. 1985 Oct;164(1):338–343. doi: 10.1128/jb.164.1.338-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGG J. E. Studies on the coccoid form of ovine Vibrio fetus I. Cultural and serologic investigations. Am J Vet Res. 1962 Mar;23:354–358. [PubMed] [Google Scholar]
- Palmer S. R., Gully P. R., White J. M., Pearson A. D., Suckling W. G., Jones D. M., Rawes J. C., Penner J. L. Water-borne outbreak of campylobacter gastroenteritis. Lancet. 1983 Feb 5;1(8319):287–290. doi: 10.1016/s0140-6736(83)91698-7. [DOI] [PubMed] [Google Scholar]
- Rollins D. M., Coolbaugh J. C., Walker R. I., Weiss E. Biphasic culture system for rapid Campylobacter cultivation. Appl Environ Microbiol. 1983 Jan;45(1):284–289. doi: 10.1128/aem.45.1.284-289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Singleton F. L., Attwell R. W., Jangi M. S., Colwell R. R. Influence of salinity and organic nutrient concentration on survival and growth of Vibrio cholerae in aquatic microcosms. Appl Environ Microbiol. 1982 May;43(5):1080–1085. doi: 10.1128/aem.43.5.1080-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., McDermott K. T., Little J. R., Wells J. G., Blaser M. J. Campylobacter enteritis from untreated water in the Rocky Mountains. Ann Intern Med. 1983 Jul;99(1):38–40. doi: 10.7326/0003-4819-99-1-38. [DOI] [PubMed] [Google Scholar]
- Vogt R. L., Sours H. E., Barrett T., Feldman R. A., Dickinson R. J., Witherell L. Campylobacter enteritis associated with contaminated water. Ann Intern Med. 1982 Mar;96(3):292–296. doi: 10.7326/0003-4819-96-3-292. [DOI] [PubMed] [Google Scholar]
- Wang W. L., Luechtefeld N. W., Blaser M. J., Reller L. B. Effect of incubation atmosphere and temperature on isolation of Campylobacter jejuni from human stools. Can J Microbiol. 1983 Apr;29(4):468–470. doi: 10.1139/m83-075. [DOI] [PubMed] [Google Scholar]
- Weiss E., Westfall H. N. Substrate utilization by Legionella cells after cryopreservation in phosphate buffer. Appl Environ Microbiol. 1984 Aug;48(2):380–385. doi: 10.1128/aem.48.2.380-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]





