Abstract
The central role that tumor antigen-derived peptides play in induction of antitumor immunity makes them ideal candidates for peptide-based cancer vaccines. We have demonstrated that “transloading” is an efficient strategy for importing short peptide ligands into antigen-presenting cells in vitro. Postulating that the transloading procedure might effect peptide uptake by antigen-presenting cells in vivo as well, we tested this approach for the generation of peptide-based cancer vaccines. In the P815 mastocytoma system, we vaccinated mice by s.c. injection of a single, known natural peptide derived from JAK-1 kinase. Whereas vaccination with peptide alone or mixed with incomplete Freund’s adjuvant was ineffective, application of the peptide in conjunction with the polycation poly-l-lysine protected a significant number of animals against tumor challenge. Dependent upon the type of poly-l-lysine applied, protection against tumor take was comparable to that achieved with irradiated whole-cell vaccines, genetically modified to secrete granulocyte–macrophage colony-stimulating factor. In the murine melanoma M-3, a combination of four putative tumor antigen-derived peptides was tested as a cancer vaccine. Administered in combination with polycations, these peptides evoked potent antitumor immunity that could not be obtained with the peptides alone or peptides emulsified in incomplete Freund’s adjuvant. However, peptide–polycation vaccines applied to the M-3 model were not as efficient as cellular control vaccines, consisting of irradiated interleukin 2 or granulocyte–macrophage colony-stimulating factor-secreting tumor cells.
Keywords: immunization/adjuvant/transloading/polyarginine/polylysine
Despite the great progress made in the treatment of certain human malignancies, the overall cancer mortality rate is still increasing. For the case of malignant melanomas of the skin, mortality in the United States has increased by 34.1% over the last 20 years (1). Although the systemic administration of high doses of recombinant interferon α has recently been registered for the treatment of malignant melanomas, this clinical intervention requires careful selection of patient types, and in mice, depending on the genetic background, it can have tumor-promoting activities (2–5). In contrast, cytokine-secreting autologous cellular cancer vaccines directed against melanomas are well tolerated (unpublished results), but their efficacy for the treatment of human cancers remains to be established. One major drawback of autologous cellular vaccines is that, in an appreciable portion of patients, tumor cells cannot be grown in vitro, a precondition for the preparation of cellular vaccines. Also, generation of the vaccines takes time in a disease where rapid vaccination is desirable. Through the pioneering work mainly of Boon and associates (6–9), we know that malignant melanomas present mainly in the major histocompatibility complex (MHC) class I-context tumor antigen (TA)-derived peptides, peptides that can be easily synthesized by standard technology. The idea of using such peptides for the generation of antitumor immunity in patients has already been tested with encouraging results (10). One difficulty for peptide-based vaccines is their rather poor immunogenicity (11), so appropriate adjuvants are needed to make them more reactive and elicit a T cell response.
We have demonstrated that a number of polycations of appropriate length and concentration, among them poly-l-lysine (pLys) and poly-l-arginine (pArg), are powerful adjuvants for transferring small peptides as the prototype for TA-derived peptides into antigen-presenting cells (APCs) (12) and tumor cells (13). In the latter case, it has been demonstrated that xenogenization of tumor cells with small immunogenic peptides elicits a powerful antitumor immunity in an MHC class I-dependent manner (13). We now show that s.c. but not i.p. coinjection of polycations with known or putative TA peptides protects mice from tumor take, i.e., that these procedures provide novel powerful cell-free tumor vaccines. Also, transloading of TA-derived peptides elicits demonstrable cellular immunity, which is thought to bode well for tumor rejection. In contrast, injection of peptides alone or peptides in combination with incomplete Freund’s adjuvant (IFA) affords no significant protection.
MATERIALS AND METHODS
Polycations.
pLys and pArg with different degrees of polymerization (dp) as indicated (see below) were obtained from Sigma.
Peptide Synthesis.
Peptides were synthesized on a peptide synthesizer (Applied Biosystems, model 433 A with feedback monitoring) using an aminomethylated polystyrene resin with p-carboxytrityalcohol linker (PepChem, Tübingen, Germany) as solid phase with the Nα-9-fluorenylmethoxycarbonyl strategy [2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate activation, Fastmoc, 0.25-mmol scale]. Peptides were dissolved in 1 M triethylammonium acetate (pH 7.3) and purified by reversed-phase chromatography on a Vydac (Hesperia, CA) C 18 column (model 218ATP54). Peptide identity was confirmed by time-of-flight mass spectrometry on Lasermat (Finnigan-MAT, San Jose, CA). The peptide sequence used in the P815 mastocytoma system was SYFPEITHI, which is H-2Kd-restricted and derived from tyrosine kinase JAK-1 (amino acids 355–363; refs. 14–16). For the M-3 murine melanoma system, four putative H-2Kd peptide ligands were used at 25 μg of each peptide per vaccine. Two were deduced from tyrosinase (17) and tyrosinase-related protein 1 (TRP-1; ref. 18). The sequences of the tyrosinase peptides were YYVSRDTLL (amino acids 128–136) and PYLEQASRI (amino acids 468–474). The sequences for TRP-1 peptides were RYAEDYEEL (amino acids 521–529) and YYSVKKTFL (amino acids 193–201).
Synthesis of Fucose-Modified pLys (fpLys).
pLys with dp ≈200 lysine residues (pLys200; 10 mg, 0.25 μmol) in 200 μl of Hepes (0.5 M, pH 8.5) was mixed with 8.5 mg (27 μmol) of β-l-fucopyranosylphenyl-isothiocyanate (Sigma) in 200 μl of dimethyl sulfoxide and reacted at room temperature for 2 hr. The product was purified by gel chromatography on Sephadex G25 (Pharmacia), yielding 0.18 μmol of pLys modified with 13.6 μmol of fucose.
Tumor Cell Culture and Generation of Cellular Vaccines.
The Cloudman S91 melanoma cells, clone M-3 (H2d), and mastocytoma P815 (H-2d) were purchased from American Type Culture Collection and cultured in high-glucose DMEM (Life Technologies, Paisley, Scotland) supplemented with 10% fetal calf serum (FCS)/2 mM l-glutamine/20 μg/ml gentamycin. Cells were routinely tested for mycoplasma contamination by a PCR-based mycoplasma detection kit (Stratagene). Transiently cytokine-secreting cellular vaccines were generated by the adenovirus-enhanced transferrinfection (AVET) technology, using the murine interleukin 2 (IL-2) and granulocyte–macrophage colony stimulating factor (GM-CSF) expression vectors and the AVET protocol as described (19). In the M-3 system, expression levels were adjusted to optimal values (IL-2, 1000–2000 units/105 cells; GM-CSF, ≥10 ng/105 cells). Constitutively GM-CSF-secreting P815 cells were generated upon Transfectam-mediated cotransfection of 10 μg of pWS–GM-CSF (ref. 19; linearized with EcoRI) and 2 μg of pRSVneo (linearized with XmnI; ref. 20) according to the manufacturer’s protocol (Promega). Cells (106) were transfected in 2 ml of serum-free DMEM for 4 hr, then 8 ml of DMEM/10% FCS was added. After 24 hr, transfected cells and untransfected control cells were transferred into 10 ml of fresh DMEM/10% FCS containing 1 mg/ml G418 (Sigma). After 2 days, when most of the cells in an untransfected group were dead, G418 was reduced to the final concentration of control cells (500 μg/ml). After 10 days, a stable pool of cells was generated, producing ≈10 ng of GM-CSF per 106 cells per 24 hr. The same amount of GM-CSF was secreted by P815 cells after transient AVET-mediated gene delivery. In both systems, P815 and M-3, 105 irradiated cells (80 Gray) were applied per vaccination.
Peptide Vaccines.
If not indicated otherwise, a total amount of 100 μg of peptide(s) was applied per animal. When polycations were used as adjuvants, peptides and polycations were incubated together for 3 hr at room temperature before injection. Per 100 μg of peptide, 7.5 μg of polycation was admixed as adjuvant except for pLys (dp ≈ 16), of which 37.5 or 75 μg was used.
Animal Experiments.
DBA/2 mice (H-2d, 6–12 weeks old) were obtained from Charles River Wiga GmbH (Sulzfeld, Germany). Groups of 8–10 animals were immunized three times in a weekly interval under halothane anaesthesia by s.c. or i.p. injection. When prophylactic treatment of naive mice was applied, contralateral challenge with viable tumor cells was administered 1 week after the last immunization. The challenge doses were 105 cells per animal (≈100 times the minimal tumorigeneic dose) in the M-3 model and 104 cells per mouse for the P815 model (>5 times the minimal tumorigeneic dose). In the therapeutic M-3 model, contralateral vaccination was started 5 days after s.c. experimental metastases were set by injection of 104 viable M-3 cells. All cellular and cell-free vaccines were applied in a total volume of 100 μl per animal. Animals were inspected daily. Tumor development was scored weekly and followed up for the indicated time periods.
Measurement of T Cell Activation After Immunization.
Single-spleen cell suspensions were prepared from vaccinated or naive animals, followed by lysis of erythrocytes with hypotonic buffer (0.15 M NH4Cl/1 mM KHCO3/0.1 mM EDTA, pH 7.4). Adherent cells were removed by incubating 3 × 106 splenocytes per ml of DMEM/10% FCS in Petri dishes (Nunc) for 90 min at 37°C. Nonadherent cells were harvested by gentle pipetting, and 2 × 105 of them were cocultured with 1 × 103 parental M-3 cells. Cells were cultured in 200 μl of DMEM containing 10% FCS (PAA, Linz, Austria), 2 mM l-glutamine, and 20 μg/ml gentamycin in 96-well flat-bottom tissue culture plates. On day 3, 50 μl of supernatant was harvested and interferon γ (IFN-γ) content was measured by ELISA using R4–6A2 as capture antibody and biotinylated A18.17.24 for detection as described (21).
RESULTS
Peptide-Based Cancer Vaccines for the P815 Mastocytoma in a Prophylactic Model.
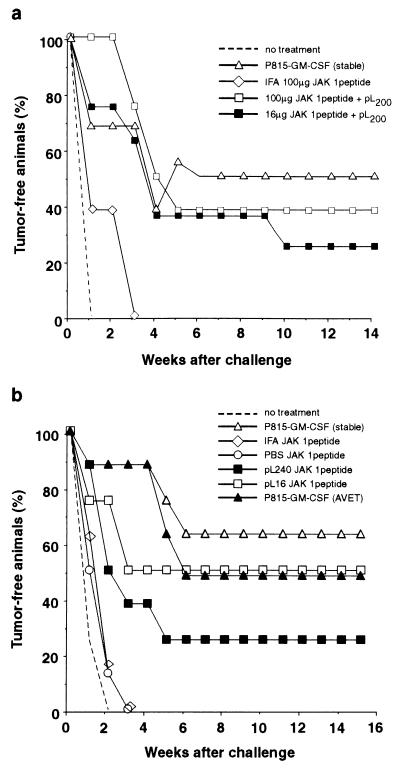
In prophylactic models, vaccination is performed before tumor challenge, whereas in the therapeutic model, tumor inoculation precedes vaccination. In the murine mastocytoma cell line P815, an unusually large proportion of approximately 5% of the MHC class I molecules in the H-2Kd haplotype present the single peptide SYFPEITHI (14, 15). This peptide displayed prominently on cancer cells is derived from murine tyrosine kinase JAK-1. We chose this prominent “self”-epitope, (14, 15) to test the peptide vaccine approach in the P815 tumor model. Unaltered self-antigens may be recognized by the immune system as TAs if inappropriately expressed, as was recently shown for tyrosinase (50). Mice were vaccinated three times at weekly intervals and, 1 week after the last vaccination, were subjected to a contralateral challenge with 104 live tumor cells. Animals vaccinated with irradiated P815 cells, stably transfected to secrete GM-CSF, were used as positive controls. Either 16 μg or 100 μg of the JAK-1 peptide was injected together with a transferrin-coupled pLys200 (22). One group of animals was applied with 100 μg of peptide per mouse emulsified in IFA. As shown in Fig. 1a, this latter treatment did not confer antitumor protection because all animals developed tumors rapidly with only a small delay as compared with untreated animals. However, when the JAK-1 peptide was applied with pLys, antitumor protection was achieved. Two of eight animals were protected in the group that received 16 μg of the peptide, and three of eight animals were protected when 100 μg was applied. Treatment with the cellular, GM-CSF-producing vaccine resulted in a protection efficiency of 50% (Fig. 1a). In a second experiment in the P815 mastocytoma model, two unmodified pLys of different chain lengths were compared, pLys16 and pLys240. Peptide control groups were injected with 100 μg of peptide per mouse; the peptide was either dissolved in PBS or emulsified in IFA. Two GM-CSF-secreting cellular control vaccines were used. The stably transfected P815 cells and a second vaccine generated by the AVET transient transfection protocol (23) were used as a positive control. Both whole-cell vaccines conferred similar protection efficiencies with four of eight and five of eight animals (Fig. 1b). The peptide-based vaccines, consisting of peptide only or peptide admixed with IFA, showed no protection; all animals developed tumors rapidly upon challenge. However, when applied in conjunction with pLys, the peptide vaccine did protect animals against tumor challenge. Two of eight animals were protected when the pLys240 was used, and four of eight animals were protected when pLys16 was used. These results show that a single peptide, when applied with pLys as adjuvant, can evoke efficient antitumor protection comparable to that of one of the most potent cytokine-secreting whole-cell vaccines that has become the gold standard for antitumor vaccination (19, 24).
Figure 1.
Vaccination of DBA/2 mice against P815 tumor challenge with the JAK-1 peptide SYFPEITHI. (a) The indicated amounts of peptide were administered per animal. The ratio of peptide to transferrin-coupled pLys200 referenced on pLys was 6.25:1 (wt/wt). (b) Vaccination with JAK-1 peptide and two pLys with dp = 16 (pLys16) or dp = 240 (pLys240). Per animal, 100 μg of peptide was applied in conjunction with 75 μg of pLys16 or 7.5 μg of pLys240.
Peptide-Based Cancer Vaccines for Murine Melanoma M-3 in a Prophylactic Model.
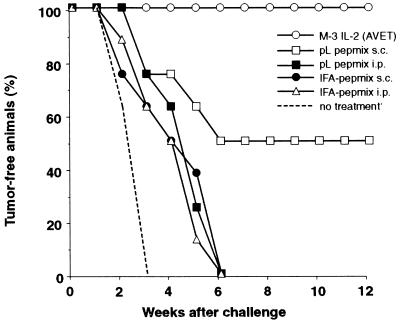
Melanocyte differentiation antigens like tyrosinase, TRP-1, or gp-100 in human melanoma have been found to be targets for specific cytotoxic T lymphocytes, and cytotoxic T lymphocyte epitopes have been identified (25–30). The murine M-3 melanoma (H-2d; ref. 31) expresses tyrosinase and TRP-1, as determined by reverse transcription PCR (ref. 32 and unpublished results). Since neither cytotoxic T lymphocyte epitopes nor immunodominant TA peptides had been identified in this tumor model, we decided to use a mixture of four putative H-2Kd-restricted peptide ligands. The selection of the peptide motifs was based on requirements for H-2Kd-restricted anchor amino residues as described (16, 33–36). Two of the peptides were derived from tyrosinase, and the other two from TRP-1 (see Materials and Methods). In a first experiment, the peptide mix was applied to vaccinate naive DBA/2 mice against tumor challenge (Fig. 2). A cellular vaccine, consisting of irradiated M-3 cells, AVET-modified to produce optimal amounts of IL-2 (19), has been shown to induce very potent antitumor immunity and was therefore chosen as positive control. As for the P815 experiment (Fig. 1), three vaccinations were applied in weekly intervals, followed by a s.c. challenge 1 week after the last vaccination. Four groups of animals were applied with the peptide mix-based vaccine. Two groups received the peptides emulsified in IFA either s.c. or i.p. The other two groups were administered with the peptides in conjunction with pLys240 via the s.c. or the i.p. route. As shown in Fig. 2, the peptide mix in conjunction with the pLys protected a significant number of animals (50%) against tumor challenge of 105 live tumor cells as compared with untreated animals, in which solid tumors formed quite rapidly. Interestingly, the peptides conferred antitumor protection in conjunction with pLys after s.c. vaccination. When applied i.p., the peptide–pLys mix was as ineffective as the IFA-based vaccines. Here, tumor challenge was not rejected, and tumors grew with only a small delay compared with untreated control animals. These results demonstrate that the tyrosinase/TRP-1-derived peptide vaccines can induce antitumor protection when administered s.c. in conjunction with pLys. However, this vaccine is only as half as efficient as the IL-2-based cellular vaccine, which, consistent with recent reports, protects up to 100% of the animals against the tumor challenge with 105 live M-3 cells (31, 37).
Figure 2.
Vaccination of DBA/2 mice against M-3 melanoma challenge with a combination of four peptides (see Materials and Methods) and pLys240. A total amount of 100 μg of peptide mix was applied per animal; the mix consisted of equal amounts of each peptide. pLys240 was used as in Fig. 1b.
Peptide-Based Cancer Vaccines for Melanoma M-3 Cells in a Therapeutic Model.
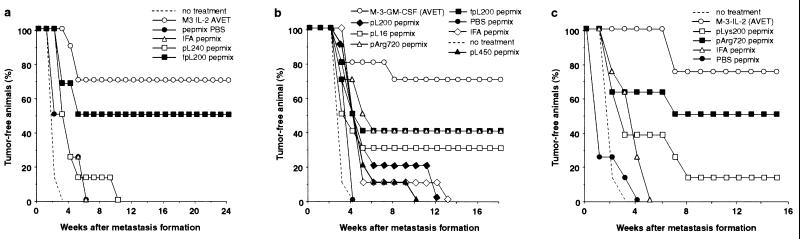
We next tested our peptide–pLys approach under more stringent experimental conditions for the treatment of a pre-existing M-3 “micrometastasis” established by s.c. injection of 104 live tumor cells 5 days before the onset of the vaccination cycle. Because the i.p. vaccination was found to be ineffective in the prophylactic treatment (Fig. 2), we decided to vaccinate only via the s.c. route. Control groups received the tyrosinase/TRP-1-derived peptide mix dissolved in PBS without adjuvant or with IFA. Unmodified pLys240 was applied as an adjuvant. Receptors for the binding of carbohydrate-terminated glycoproteins, involved in the phagocytosis of pathogens, are widely expressed on phagocytotic cells of the skin (38). We therefore reasoned that it might be possible to improve targeting of such cells with fpLys. Therefore, peptides mixed with fpLys200 were also administered. For comparison, a group receiving the IL-2-based cellular vaccine was included in this experiment. As shown in Fig. 3a, the pLys–peptide vaccine was effective for the treatment of pre-existing M-3 deposits. However, a significant cure rate was achieved only with the fpLys. With this treatment, 50% of the animals rejected the experimental metastases, a result that compares very favorably with the IL-2-based vaccine that cured 70% of the mice in this instance. Unmodified pLys–peptide vaccine, which is efficient against tumor challenge in the prophylactic model (Figs. 1 and 2), was not very effective in the therapeutic model. Compared with the untreated control group, it achieved only a delay in tumor onset, not complete rejection. When treated with peptides alone or peptides in IFA, all mice developed tumors without delay.
Figure 3.
Cure of M-3 micrometastases-bearing mice with four peptides and polycations as adjuvant. Peptides were used as described in Fig. 2. The following amounts of polycations were used per 100 μg of peptide mix: pLys16, 37.5 μg; fpLys200, pLys240, pLys450, and pArg720, 7.5 μg. Ten animals per group were treated in a and b, and eight animals were treated in the groups of c.
We further studied the effect of alterations and modifications of the polycation on the antitumor efficiency of our peptide-based vaccine in the M-3 metastasis model (Fig. 3b). In addition to unmodified pLys200 and fpLys200, unmodified pLys16, pLys450, and a different polycation, polyarginine pArg720, were tested. As a positive control, a cellular vaccine secreting GM-CSF was applied (32). Again, control groups receiving the peptide mix in IFA or without any adjuvant were also included. As expected from the experiment shown in Fig. 3a, the best peptide-mediated efficacy was achieved when fpLys was applied as adjuvant (Fig. 3b). In this group, 40% of the animals rejected the metastases compared with 30% in the group administered with pLys16. In all other peptide groups, there was only a delay in tumor onset as compared with untreated animals, except for peptides, which were admixed with pArg. Interestingly, this unmodified polycation was as effective as the fpLys, enabling the rejection of the M-3 deposits in 4 of 10 animals. Fig. 3c shows the reproduction of this pArg effect in an independent experiment. Again, vaccination with tyrosinase/TRP-1-derived peptides in conjunction with pArg showed antitumor efficacy in 4 of 8 treated animals. These results identify pArg as a very interesting adjuvant for the application of peptide-based cancer vaccines.
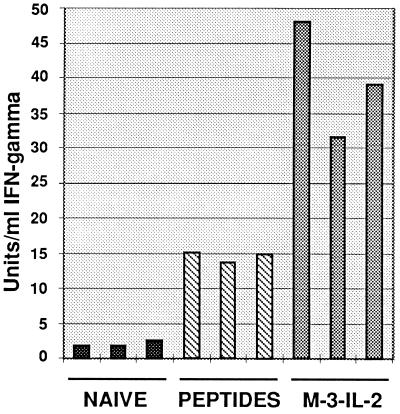
Although it is evident from the experiments described above that vaccination with a combination of peptides and polycations leads to protection of mice, we wished to address the question of whether T cells were also activated by this treatment. Cytokine secretion upon exposure of splenocytes from vaccinated animals to parental M-3 cells was taken as a surrogate marker for T cell activation (39, 40). Only splenocytes from vaccinated animals secreted substantial levels of IFN-γ (Fig. 4) upon cocultivation with M-3 cells in vitro, whereas no IFN-γ was detectable from splenocytes of naive animals.
Figure 4.
IFN-γ release in response to M-3 target cells by nonadherent splenocytes from DBA/2 mice. Twenty-four weeks after metastases formation, splenocytes of disease-free mice cured by the IL-2-secreting cellular vaccine (M-3–IL-2) or fpLys–peptide mix treatment (Fig. 3a) were used in the assay. An age-matched naive mouse was used as negative control. Triplicate measurements of IFN-γ production are shown.
DISCUSSION
Evidence from both bone marrow transplantation experiments (41) and histological investigations (32, 42, 43) in the murine system suggests the following working hypothesis for the events leading to the generation of cellular antitumor immunity. In the case of cellular cytokine-secreting irradiated vaccines, macrophage-like cells invade the vaccine deposit of irradiated cells and phagocytose the tumor cells within a relatively short period of days. It is proposed that, as a next step, APCs charged with ingested tumor cells present TA peptides to naive T cells in the draining lymph node, leading to an immune cascade and formation of activated tumor-specific T cells which then patrol the tissues and eradicate, in the murine system, distant deposits of a live tumor challenge and, in the human situation, distant micrometastases. From mouse studies, it became clear that the level of IL-2 secretion by the cellular vaccines is of paramount importance for the elicitation of an antitumor response; IL-2 levels that were too low or too high were ineffective (19, 44). Both the optimal level of IL-2 secretion and the mode of tumor eradication in humans remain to be determined.
Implicit in the above working hypothesis is the tenet that TAs are recognized by mechanisms leading to a powerful cellular immunity. By presenting TA peptides by means of our transloading procedure, which rapidly and efficiently internalizes peptides into cells, we may fortuitously have met an important precondition for eliciting a cascade of events leading to the generation of T cell immunity. That in our experiments cellular antitumor immunity has been generated for both IL-2 and peptide vaccines became clear when spleen cells from protected animals were shown to stimulate high levels of IFN-γ in vitro upon incubation of splenocytes with M-3 tumor cells (Fig. 4). It is conceivable that in future such in vitro cytokine tests will provide a faster and more quantitative measurement of vaccine efficacy than determinations of tumor take in a group of animals. Investigation of the P815 system yielded similar results (data not shown).
The vaccination effect, as determined by the number of animals protected from tumor incidence, was considerably more pronounced in the prophylactic model than in the therapeutic model, the latter being possibly more representative of the situation found in the human disease. In the therapeutic model, there is time for the deposit of live tumor cells to be established in naive mice in the absence of antitumor immunity, and such deposits that resemble micrometastases may be more difficult to eradicate than a bolus of live tumor cells deposited in a mouse, in which an antitumor response has already been established by prior vaccination.
The skin is a tissue rich in macrophage-like cells, such as dendritic and Langerhans cells, which are potent APCs. We surmised that by injection of polycations, either pLys or pArg, together with known or putative TA peptides, it might be possible to engage such APCs directly to set the immune cascade in motion. Alternatively, transloading peptides might target tissue cells, which later fall victim to APCs, leading to TA presentation by a more complicated route. If APCs are the direct targets for transloading, how does a peptide transferred by endocytosis in the presence of the adjuvant pArg (12) ultimately present in an MHC class I context? This contravenes the classical tenet that antigens entering by an endosomal mechanism are presented by MHC class II molecules (into which our peptides would not fit), but recent investigations have shown that there is considerable plasticity in the processing and compartmentalization of antigens and their peptides, respectively, and their presentation on MHC molecules (45), making a class I presentation in our experiments at least plausible.
Tumor take has previously been suppressed for the 3LL lewis lung carcinoma system after injection of TA peptides with IFA (11), but in our P815 and M-3 systems, no protection was obtained with such a protocol (see above). However, it should be noted that a combination of IFA and viral immunogenic peptides can induce tolerance (46–48). With the novel modification described in this paper, vaccination proved to be effective even in the therapeutic model, provided that the appropriate polycations are used as adjuvants. To compare the potency of peptide vaccines, we ran these experiments concurrently with cytokine-secreting vaccines, which have become the gold standard for immunological cancer eradication. In the case of the P815 mastocytoma with a known prominent H-2Kd-restricted JAK-1 peptide (14, 34), vaccines at high input of the peptide (100 μg per vaccination) were nearly as good as GM-CSF-secreting whole-cell vaccines when a short pLys was employed. For the M-3 melanoma test system, protection against tumor take was less than that afforded by vaccines secreting IL-2 at the optimal level (1000 units per 24 hr and per vaccine), but in these experiments, it has not as yet been ascertained whether the peptides used were indeed potent TA peptides. The efficacy of peptide vaccines also in this system may be improved as soon as genuine immunodominant peptides have been identified.
Although we show here that transloading is efficatious for the generation of anti-tumor immunity, a similar approach could be used for other vaccination purposes. The success of the experiments depends on the nature of the polycation used. In the prophylactic model, pLys450 (not shown) was less potent than pLys200, and this result may be a consequence of the more toxic effect detected for pLys of higher molecular weight (12, 49). We have shown (12) that pArg720 in a similar concentration transfers peptides more efficiently than did pLys400 and was less toxic. Therefore, it is interesting to note that protection against tumor development was better for pArg than pLys (Fig. 3 b and c). But in all experiments, there is ample scope for improving the efficiency of peptide vaccines, e.g., by the use of modified polycations, which will allow more efficient targeting of APCs. Another avenue to follow is coinjection of peptide(s) and cytokines to recruit APCs to the site of peptide deposition. Further, the use of TA proteins in combination with polycations should be investigated because this would make vaccination less dependent on the MHC haplotype of a treated individual.
ABBREVIATIONS
- APC
antigen-presenting cell
- AVET
adenovirus-enhanced transferrinfection
- dp
degree of polymerization
- fpLys
fucose-modified poly-l-lysine
- GM-CSF
granulocyte–macrophage colony stimulating factor
- IFA
incomplete Freund’s adjuvant
- IFN-γ
interferon γ
- IL-2
interleukin 2
- MHC
major histocompatibility complex
- pArg
poly-l-arginine
- pLys
poly-l-lysine
- pLys200
pLys with a dp of 200
- TA
tumor antigen
References
- 1.Rennie J, Rusting R. Sci Am. 1996;275(3):28–30. doi: 10.1038/scientificamerican0996-56. [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood J M, Strawderman M H, Ernstoff M S, Smith T J, Borden E C, Blum R H. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Cascinelli N, Bufalino R, Morabito A, Mackie R. Lancet. 1994;343:913–914. doi: 10.1016/s0140-6736(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 4.De Maeyer Guignard J, Lauret E, Eusebe L, De Maeyer E. Proc Natl Acad Sci USA. 1993;90:5708–5712. doi: 10.1073/pnas.90.12.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solal Celigny P, Lepage E, Brousse N, Reyes F, Haioun C, Leporrier M, Peuchmaur M, Bosly A, Parlier Y, Brice P, Coiffier B, Gisselbrecht C. N Engl J Med. 1993;329:1608–1614. doi: 10.1056/NEJM199311253292203. [DOI] [PubMed] [Google Scholar]
- 6.Boon T. Adv Cancer Res. 1992;58:177–210. doi: 10.1016/s0065-230x(08)60295-x. [DOI] [PubMed] [Google Scholar]
- 7.Boon T, Cerottini J C, Van den Eynde B, van der Bruggen P, Van Pel A. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 8.van Pel A, van der Bruggen P, Coulie P G, Brichard G V, Lethe B, van den Eynde B, Uyttenhove C, Renauld J C, Boon T. Immunol Rev. 1995;145:229–250. doi: 10.1111/j.1600-065x.1995.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 9.Van der Eynde B, Brichard V G. Curr Opin Immunol. 1995;7:674–681. doi: 10.1016/0952-7915(95)80076-x. [DOI] [PubMed] [Google Scholar]
- 10.Marchand M, Weynants P, Rankin E, Arienti F, Belli F, et al. Int J Cancer. 1995;63:883–885. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 11.Mandelboim O, Vadai E, Fridkin M, Katz Hillel A, Feldman M, Berke G, Eisenbach L. Nat Med. 1995;1:1179–1183. doi: 10.1038/nm1195-1179. [DOI] [PubMed] [Google Scholar]
- 12.Buschle M, Schmidt W, Zauner W, Mechtler K, Trska B, Kirlappos H, Birnstiel M L. Proc Natl Acad Sci USA. 1997;94:3256–3261. doi: 10.1073/pnas.94.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt W, Steinlein P, Buschle M, Schweighoffer T, Herbst E, Mechtler K, Kirlappos H, Birnstiel M L. Proc Natl Acad Sci USA. 1996;93:9759–9763. doi: 10.1073/pnas.93.18.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee H G. Nature (London) 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 15.Harpur A G, Zimiecki A, Wilks A F, Falk K, Rotzschke O, Rammensee H G. Immunol Lett. 1993;35:235–237. doi: 10.1016/0165-2478(93)90188-8. [DOI] [PubMed] [Google Scholar]
- 16.Rammensee H G, Friede T, Stevanovic S. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 17.Müller G, Ruppert S, Schmid E, Schutz G. EMBO J. 1988;7:2723–2730. doi: 10.1002/j.1460-2075.1988.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibahara S, Tomita Y, Sakakura T, Nager C, Chaudhuri B, Müller R. Nucleic Acids Res. 1986;14:2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt W, Schweighoffer T, Herbst E, Maass G, Berger M, Schilcher F, Schaffner G, Birnstiel M L. Proc Natl Acad Sci USA. 1995;92:4711–4714. doi: 10.1073/pnas.92.10.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman C, Padmanabhan R, Howard B H. Science. 1983;221:551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt E, Hoehn P, Germann T, Rude E. Eur J Immunol. 1994;24:343–347. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- 22.Wagner E, Zenke M, Cotten M, Beug H, Birnstiel M L. Proc Natl Acad Sci USA. 1990;87:3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner E, Zatloukal K, Cotten M, Kirlappos H, Mechtler K, Curiel D T, Birnstiel M L. Proc Natl Acad Sci USA. 1992;89:6099–6103. doi: 10.1073/pnas.89.13.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brichard V, Van Pel A, Wölfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker A B, Schreurs M W, de Boer A J, Kawakami Y, Rosenberg S A, Adema G J, Figdor C G. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins P F, Rivoltini L, Yannelli J R, Appella E, Rosenberg S A. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins P F, el Gamil M, Kawakami Y, Stevens E, Yannelli J R, Rosenberg S A. Cancer Res. 1994;54:3124–3126. [PubMed] [Google Scholar]
- 29.Wölfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Buschenfelde K H, Boon T. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 30.Wang R F, Robbins P F, Kawakami Y, Kang X Q, Rosenberg S A. J Exp Med. 1995;181:799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zatloukal K, Schneeberger A, Berger M, Schmidt W, Koszik F, Kutil R, Cotten M, Wagner E, Buschle M, Maass G, Payer E, Stingl G, Birnstiel M L. J Immunol. 1995;154:3406–3419. [PubMed] [Google Scholar]
- 32.Schmidt, W., Maass, G., Buschle, M., Schweighoffer, T., Berger, M., Herbst, E., Schilcher, M. & Birnstiel, M. L. (1996) Gene, in press. [DOI] [PubMed]
- 33.Rotzschke O, Falk K. Immunol Today. 1991;12:447–455. doi: 10.1016/0167-5699(91)90018-O. [DOI] [PubMed] [Google Scholar]
- 34.Rammensee H G, Falk K, Rotzschke O. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- 35.Rammensee H G, Falk K, Rotzschke O. Curr Opin Immunol. 1993;5:35–44. doi: 10.1016/0952-7915(93)90078-7. [DOI] [PubMed] [Google Scholar]
- 36.Rammensee H G. Curr Biol. 1995;7:85–96. doi: 10.1016/0952-7915(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 37.Zatloukal K, Schmidt W, Cotten M, Wagner E, Stingl G, Birnstiel M L. Gene. 1993;135:199–207. doi: 10.1016/0378-1119(93)90066-c. [DOI] [PubMed] [Google Scholar]
- 38.Lanzavecchia A. Curr Opin Immunol. 1996;8:348–354. doi: 10.1016/s0952-7915(96)80124-5. [DOI] [PubMed] [Google Scholar]
- 39.Schwartzentruber D J, Topalian S L, Mancini M, Rosenberg S A. J Immunol. 1991;146:3674–3681. [PubMed] [Google Scholar]
- 40.Kawakami Y, Eliyahu S, Delgado C H, Robbins P F, Sakaguchi K, Appella E, Yannelli J R, Adema G J, Miki T, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang A Y, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 42.Bannerji R, Arroyo C D, Cordon Cardo C, Gilboa E. J Immunol. 1994;152:2324–2332. [PubMed] [Google Scholar]
- 43.Maass G, Schmidt W, Berger M, Schilcher F, Koszik F, Schneeberger A, Stingl G, Birnstiel M L, Schweighoffer T. Proc Natl Acad Sci USA. 1995;92:5540–5544. doi: 10.1073/pnas.92.12.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fakhari H, Shawler D L, Gjerset R, Naviaux R K, Koziol J, Royston I, Sobol R E. Hum Gene Ther. 1995;6:591–601. doi: 10.1089/hum.1995.6.5-591. [DOI] [PubMed] [Google Scholar]
- 45.Jondal M, Schirmbeck R, Reimann J. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- 46.Aichele P, Brduscha Riem K, Zinkernagel R M, Hengartner H, Pircher H. J Exp Med. 1995;182:261–266. doi: 10.1084/jem.182.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aichele P, Kyburz D, Ohashi P S, Odermatt B, Zinkernagel R M, Hengartner H, Pircher H. Proc Natl Acad Sci USA. 1994;91:444–448. doi: 10.1073/pnas.91.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toes R E M, Offringa R, Blom R J J, Melief C J M, Kast W M. Proc Natl Acad Sci USA. 1996;93:7855–7860. doi: 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnold L J, Dagan A, Gutheil J, Kaplan N O. Proc Natl Acad Sci USA. 1979;76:3246–3250. doi: 10.1073/pnas.76.7.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth C, Rochlitz P, Kourilsky P. Annu Rev Immunol. 1994;13:399–415. [Google Scholar]