Abstract
Expression of molecules with antiparasitic activity by genetically transformed symbiotic bacteria of disease-transmitting insects may serve as a powerful approach to control certain arthropod-borne diseases. The endosymbiont of the Chagas disease vector, Rhodnius prolixus, has been transformed to express cecropin A, a peptide lethal to the parasite, Trypanosoma cruzi. In insects carrying the transformed bacteria, cecropin A expression results in elimination or reduction in number of T. cruzi. A method has been devised to spread the transgenic bacteria to populations of R. prolixus, in a manner that mimics their natural coprophagous route of symbiont acquisition.
Insects transmit many of the diseases that affect crop plants, domestic animals, and humans. Such diseases are particularly important in the developing world. Plants produce toxic or inhibitory substances to protect themselves against insects, and in the last hundred years or so people have joined them in this effort with insecticides (1). Success has been limited. Insects have developed an array of resistance mechanisms by which they can inactivate toxic substances (2). Even biological methods, such as the sterile male techniques, have been rendered less effective by changes in insect behavior (3). In a wider sense, it is not always desirable to eliminate insect pests, because they may occupy an important niche in the food chain.
We are developing methods that reduce the competence of the insect vector to carry disease-causing microorganisms. We attempt to affect a specific property of the insect rather than the existence of the insect itself. Insects with relatively restricted diets, for instance those feeding on either blood or plant sap, usually harbor populations of symbiotic microorganisms that synthesize the needed nutrients (4). We here show that such symbiotic bacteria (symbionts) can be transformed genetically to express and release transgene products into the insect tissues that are deleterious to the disease-causing microorganisms the insect carries.
There are other methods for introducing transgenes into insects. Recently, Olson et al. (5) have generated mosquitoes that are refractory to transmitting dengue-2 virus by infecting the mosquitoes with a recombinant Sindbis virus that expresses dengue-2 antisense viral message. However, the phenotype is nonheritable to progeny. The mobile P element has long been used as a gene carrier in drosophilids, and other effective mobile elements are being studied, such as the hAT family, which may be useful for introducing foreign genes into insects of economic or medical importance (6). Mobile element insertions can be widespread and affect insect fitness (7). What attracts us to the use of transformed symbionts is the relative simplicity of the approach and absence of adverse fitness effects that can result from transformation of the insect genome. The close association between symbionts and their insect hosts also provides a method for rapid spread of the transgene throughout an insect population.
The reduviid bug Rhodnius prolixus is a vector of Trypanosoma cruzi, the causative agent of Chagas disease. Transmission of the parasite occurs at the time of a blood meal, when the bug deposits a fecal droplet on the skin of the warm-blooded host. R. prolixus harbors in its gut lumen an extracellular streptomycete symbiont, Rhodococcus rhodnii, situated in close proximity to T. cruzi. Previously, our group has transformed R. rhodnii using a shuttle plasmid containing a thiostrepton resistance marker (8). The transformed bacteria, when introduced into aposymbiotic first-instar nymphs of R. prolixus, allow sexual maturation of the insects. If left in the aposymbiotic state, such nymphs usually do not survive beyond the second molt. Thiostrepton resistant organisms were detected in the gut of these insects for the 6½-month duration of the study. Thus, recombinant symbionts can be introduced into an insect host and maintained in a stable fashion. We here report that R. rhodnii can be genetically altered to express a gene for cecropin A, a 38-amino acid residue peptide (37 amino acids plus a C-terminal glycine which is processed to an amide) that is one of a group of naturally occurring pore-forming peptides isolated from hemolymph of insects and from vertebrates (9), which are part of inducible humoral immunity. Stable expression and release of this peptide into the hind gut kills T. cruzi without apparent toxicity to bug tissues. Furthermore, we show that the coprophagic behavior of the bug can be exploited in closed colonies of insects to spread the transgene-carrying symbiont.
MATERIALS AND METHODS
Activity of Cecropin A.
Synthetic cecropin A 38 (37 amino acids plus a C-terminal glycine) prepared at The Rockefeller University (R.B.M.) was used for killing assays with Escherichia coli strain DH5αMCR, R. rhodnii strain ATCC 35071, and T. cruzi strains DM28, Gainesville and “Y.” T. cruzi strain DM28 was kindly provided by Patricia de Azambuja (Fundaçao Oswaldo Cruz, Rio de Janeiro, Brazil). “Y” strain T. cruzi was kindly provided by Norma Andrews (Yale University). Minimum bactericidal concentration was defined as the lowest concentration of cecropin A needed to produce no bacterial growth on agar plates. Lethal concentration of cecropin A was defined as the concentration required to kill 100% of parasites at 24 h. All assays were repeated five times.
Cloning cDNA for Cecropin A.
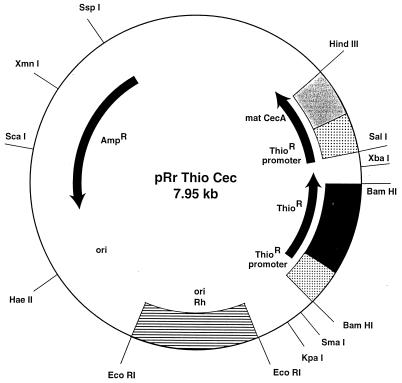
cDNA-encoding cecropin A (mat cec A) (10) was amplified by PCR with incorporation of a HindIII restriction site at the 3′ end, using the primers 5′-CCAGTTAAGCTTTTAACCCTTAGCAATCTGTGT-3′ and 5′-ATGAAATGGAAGTTATTCAAGAAG-3′. The promoter region of the thiostrepton resistance gene (ThioR) (8) was PCR-amplified with incorporation of a 5′ SalI restriction site, using the primers 5′-GCGTCGACCCGATTCGCGTCCGCCCCGGGGAG-3′ and 5′-TCGGTTGGCCGCGAGATTCCT-3′. Blunt end ligation was done to yield the ThioR promoter-mat cecA fragment. PCR amplification of the ThioR promoter-mat cecA fragment followed by cloning into SalI and HindIII sites of pRr 1.1 (8) yielded pRr ThioCec. Orientation of pRr ThioCec was confirmed using restriction enzyme mapping and subsequent DNA sequencing.
Expression of Cecropin-A in R. prolixus.
For transformation of R. rhodnii, protoplast forms of R. rhodnii were generated and transformed using pRr ThioCec as described (11). Transformants were selected using liver infusion tryptose medium containing thiostrepton at 50 μg/ml (Sigma).
For generation of aposymbiotic R. prolixus, freshly laid eggs from laboratory colonies of R. prolixus were surface-sterilized for 3 min in Clinidine solution (Clinipad, Guilford, CT), washed in sterile PBS, and allowed to hatch in sterile chambers. The emerging nymphs were shown to contain no bacteria in the gut, by plating their intestinal contents on brain heart infusion agar (Difco).
Transformed R. rhodnii were mixed with defibrinated rabbit blood (Crane Laboratories, Syracuse, NY) to a concentration of 1 × 107 bacteria per ml and fed to aposymbiotic first-instar nymphs of R. prolixus using a membrane apparatus (12).
Western blot analysis was done when five R. prolixus third-instar nymphs carrying the R. rhodnii symbiont, which had been transformed to express the cecropin A gene product, were killed and surface-sterilized using 100% ethanol for 10 min. Hind gut contents were dissected and resuspended in 200 μl of sterile PBS. Ten mililiter aliquots were used for protein electrophoresis. The proteins were separated on 10% acrylamide using the Tricine-SDS/PAGE system (13), transferred to poly(vinylidene difluoride) membranes (Bio-Rad), and developed using a 1:1000 dilution of polyclonal serum from rabbits immunized with synthetic cecropin A (Cocalico Biologicals, Reamstown, PA).
E. coli Killing Assays.
The whole gut of each of five third-instar nymphs of R. prolixus was dissected out, and each gut was homogenized in 100 μl of sterile PBS. Ten microliters of each homogenate was incubated at 37°C for 12 h with 20 μl of strain DH5αMCR of E. coli (1 × 105 bacteria/ml). The incubation mixture was diluted with 970 μl of sterile Luria–Bertani medium and plated on Luria–Bertani agar plates. Five replicates of each killing assay were performed.
T. cruzi Killing Assays.
The effects of in vivo production of cecropin A on numbers of metacyclic trypomastigotes in the insect hind gut was determined over a 90-day period. Fourth-instar nymphs were infected with strain DM28 T. cruzi. This Brazilian strain of T. cruzi is pathogenic to man. Decomplemented human blood was mixed with epimastigote forms to a concentration of 1 × 105 parasites/ml (14). Insects were allowed to engorge on the infected blood. Subsequent blood meals were administered 3, 6, and 9 weeks after initial infection with the parasites.
Seven adult insects from each experimental group were killed 14 days after the final blood meal. The hind gut of each insect was dissected and homogenized in 100 μl of sterile PBS. Numbers of metacyclic trypomastigotes, the infective form of T. cruzi, were counted without staining, using a Neubauer hemocytometer.
RESULTS
Activity of Cecropin A and Stability of pRr ThioCec.
Cecropin A has a wide range of antimicrobial activity (15). We have observed very little effect of this peptide on R. rhodnii (see Fig. 1) or on gut cells of R. prolixus. We also have demonstrated activity of synthetic cecropin A against several strains of T. cruzi (Fig. 1), including strain DM28, a common human pathogen.
Figure 1.
Concentrations of synthetic cecropin A lethal for E. coli, the R. rhodnii symbiont, and three strains for T. cruzi. Values for E. coli and R. rhodnii are minimum bactericidal concentrations (MBC). The values for T. cruzi (epimastigote forms derived from cell culture) are concentrations needed to kill 100% of parasites at 24 h of incubation (LC). The DM28 strain was used for our in vivo experiments and is a common human Chagas disease pathogen in Brazil.
We have incorporated the gene encoding cecropin A into the shuttle vector, pRr1.1, to yield the new shuttle plasmid pRr Thio Cec. This plasmid (Fig. 2) was used to transform exponential phase R. rhodnii. Ten-day-old colonies of transformed R. rhodnii were inoculated into LIT liver infusion tryptose medium containing thiostrepton (50 μg/ml). Cecropin A expression was shown by Western blots using bacterial lysates and culture supernatant from 10- to 14-day-old colonies (data not shown).
Figure 2.
The shuttle plasmid pRr Thio Cec.
The stability of pRr Thio Cec was demonstrated by maintaining the genetically altered bacteria in log phase growth for 20 generations in liver infusion tryptose medium without thiostrepton. Samples were taken every 4 days, i.e. approximately every two generations, and replica-plated on brain heart infusion agar with and without thiostrepton (8). Colony counts showed overall plasmid loss of 0.5% per generation. Thus, pRr Thio Cec is a relatively stable plasmid in the absence of antibiotic selection and is suitable for reintroduction into insects. We have used R. rhodnii transformed with this plasmid in our work.
Expression of Recombinant Cecropin A in R. prolixus.
Aposymbiotic colonies of R. prolixus were established. R. rhodnii transformed with the shuttle plasmid pRr Thio Cec were then introduced into first-instar nymphs via the first blood meal. A control group of aposymbiotic R. prolixus containing wild-type R. rhodnii also was established. Both colonies, each consisting of 35 insects, were maintained on monthly feeds of human blood. Growth rates and stage specific mortality of the two groups were similar; in each group approximately 50% of the insects reached sexual maturity at 6 months. In our insectary, mortality of aposymbiotic F1 nymphs of R. prolixus to the adult stage is approximately 50 percent. Insects that fail to feed on a membrane apparatus and do not obtain bacterial symbionts via the blood meal die after the second molt. Thus, mortality in this group of insects is higher than in F1 nymphs, which are not aposymbiotic.
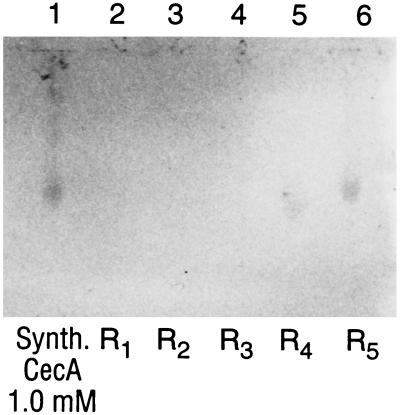
Five nymphs at the third-instar stage from each group were sampled for recombinant cecropin A in the hind gut using Western blots. Cecropin A could be detected in 2 of 5 insects bearing genetically transformed symbionts (Fig. 3). Cecropin A could not be detected in any of the insects containing wild-type R. rhodnii. These data, taken alone, could suggest low efficiency of cecropin A expression in this system. However, our subsequent bioassays indicate that Western blot is a relatively insensitive technique to screen for presence of cecropin A.
Figure 3.
Western blot. Lane 1, synthetic cecropin A, 5 μl of a 1.0 mM aqueous solution (4.1 kDa). Lanes 2–6, hind gut preparations from five third-instar nymphs of R. prolixus (R1–R5) carrying R. rhodnii, which has been transformed to express the cecropin A gene product. Bioassays (Fig. 4) showed that all five specimens (R1–R5) had significant biological activity attributable to cecropin A, though only R4 and R5 had sufficient cecropin A to be detected in our Western blot system.
In Vivo Effects of Recombinant Cecropin A Expression.
Lytic function of the recombinant cecropin A transgene product was tested, using an E. coli-killing assay. Strain DH5αMCR was used, in which the minimum bactericidal concentration of cecropin A is 23 μM. Fig. 4 shows that antimicrobial activity was present in gut preparations of all 5 transgene-bearing insects. A minimum bactericidal concentration of cecropin A was present in 3 of the 5 tested gut samples. Intestinal preparations taken from insects carrying untransformed R. rhodnii showed no lytic effect on E. coli.
Figure 4.
Assays for biological activity. GA, samples from R. prolixus nymphs carrying the R. rhodnii symbiont transformed to express the cecropin A gene product. WT, samples from R. prolixus nymphs carrying native symbionts. Synthetic CecA, control incubations with 10 μl sterile Luria–Bertani medium. Gut contents from insects carrying the cecropin A-expressing symbionts markedly inhibited the growth of E. coli.
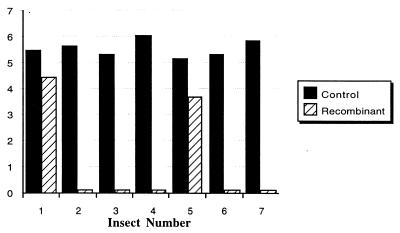
Results of the in vivo T. cruzi killing assay (Fig. 5) confirm the antiparasitic effects of recombinant cecropin A. The concentration of metacyclic trypomastigotes in the control group of insects (1.4 × 105 − 1.1 × 106 parasites/ml) is consistent with data reported by others for naturally occurring gut numbers of these parasites (16), and indicates successful infection of the insects by trypanosomes. Striking results were obtained from the transgene-bearing insects. In 5 of 7 insects in this group metacyclic trypomastigotes were not seen. Epimastigotes were absent as well, indicating complete lysis of trypanosomes. Metacyclic trypomastigotes were seen in 2 of 7 insects, at concentrations of 2.7 × 104 and 5.0 × 103 parasites/ml, respectively.
Figure 5.
Number of T. cruzi in the hind gut of R. prolixus in control (black bars) R. prolixus carrying native symbiotic R. rhodnii in the hind gut, and (hatched bars) recombinant group where R. prolixus carry genetically modified R. rhodnii, which express the gene for cecropin A peptide. Unstained metacyclic trypomastigotes were counted, using a Neubauer hemocytometer, and the count number is expressed as log10. All values are the mean of four measurements. No trypanosomes were seen in recombinant insects 2, 3, 4, 6, and 7, and the hatched bars in these columns indicate that counts were performed.
Spreading the Transgene.
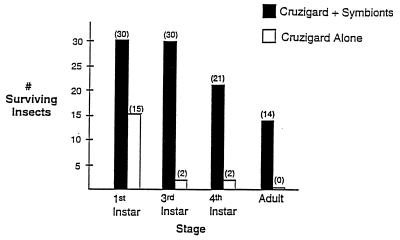
An approach is required for spreading the transgene through insect populations. A delivery system allowing dispersal of recombinant genetic material without adverse environmental effects is preferable. Two biologic features of R. prolixus may be used. These insects are primarily domestic, stably associated with their domestic animal hosts. Coprophagy, i.e. probing of fecal droplets, allows acquisition of bacterial symbionts by these insects. Emerging first-instar nymphs are transiently aposymbiotic. Probing of the egg shell surface or of fecal droplets deposited by other insects results in establishment of gut symbiont populations. We have exploited these two features to create an artificial feces preparation, termed CRUZIGARD, which we are testing in closed colonies of R. prolixus. Aposymbiotic first-instar nymphs, when placed in a chamber containing CRUZIGARD impregnated with transformed R. rhodnii, show approximately 50% survival to the sexually mature adult stage, a rate comparable to what we see in aposymbiotic nymphs exposed to natural feces containing wild-type symbionts. Thiostrepton resistance can be detected in symbiont colonies obtained from these insects, suggesting that symbionts were acquired from the artificial feces. Aposymbiotic first-instar nymphs placed in a chamber containing CRUZIGARD not impregnated with symbionts show 100% mortality to the adult stage, with most mortality occurring before the second molt (Fig. 6).
Figure 6.
Survival of aposymbiotic first-instar nymphs of R. prolixus exposed to CRUZIGARD. Thirty nymphs were exposed to CRUZIGARD impregnated with genetically transformed R. rhodnii. Fifteen nymphs were exposed to CRUZIGARD containing no added bacteria. Aposymbiotic first-instar nymphs exposed to natural feces in our insectary experience, on average, 50% mortality to the adult stage.
DISCUSSION
This is the first description of the modulation of arthropod vector competence using genetically altered symbiotic bacteria. We also have a potential method for spreading transgenic symbionts throughout an insect population. This method appears to work under laboratory conditions using a small number of insects. Our next task is to see if it works in natural settings with larger numbers. It is now at the stage where cage trials can be carried out to determine whether or not vector transmission of T. cruzi is reduced or eliminated.
Development of infective metacyclic trypomastigotes is unhindered in insects carrying wild-type R. rhodnii, whereas insects carrying bacteria transformed with the cecropin A gene had elimination of parasites in five insects and a 1–2 log reduction in the remaining two insects. Because all insects engorged on blood containing the same high number of trypanosomes, it is unlikely that there were large variations in inoculum dose of parasites. All insects show a reduction or elimination of T. cruzi, yet in two insects approximately 0.1% to 1.0% of trypanosomes remained alive. This is probably due to variations in the lysis of symbionts and/or the level of expression of cecropin A. Cecropin A makes holes in the membrane of T. cruzi, and complete lysis is probably both a dose- and time-dependent process. We do not know whether or not the remaining trypanosomes are still infective, but this is under investigation.
Constitutive expression of recombinant cecropin A by symbiotic bacteria in the hind gut of R. prolixus is lytic to developing trypanosomes. The gene for cecropin A is stable in the absence of antibiotic selection. Transformed bacteria appear to maintain a stable relationship with the insect host. Toxicity of the recombinant peptide toward gut flora or insect tissues does not appear to be a problem, given the similar development of the transgene-bearing nymphs with respect to the control group of insects carrying wild-type R. rhodnii.
Activity of gut extracts against both E. coli and strain DM28 T. cruzi was uniform, in contrast to Western blot detection of cecropin A, which was positive in only two of five insects. Thus, the sensitivity of bioassays appears to be superior to the sensitivity of Western blot.
In a closed environment, an artificial substrate impregnated with modified symbionts has been used to disperse transgenes. In domestic reduviids, such as R. prolixus in Central America, where a limited number of bugs probe feces in a limited area, such as a house or a hut, fine dispersion of simulated feces might spread the transgene effectively. We are now testing this method under closed conditions that simulate a natural environment.
Fitness of the transformed symbionts with respect to wild-type bacteria is an important factor when devising a gene-spreading strategy. We previously have shown using the shuttle plasmid pRr 1.1 that transformed R. rhodnii effectively compete in a laboratory setting with untransformed R. rhodnii to establish infection in aposymbiotic R. prolixus (C.B.B., unpublished data). We are now setting up, in cages, simulated field studies to test the competitive fitness of R. rhodnii transformed with pRr Thio Cec with respect to natural bacterial populations.
Development of resistance among target populations to the transgenic product should be considered. Cecropin A, if expressed alone in the insect gut, could possibly select for resistant forms of T. cruzi. For this reason, we are focusing attention on other potential genes that could confer refractoriness, such as genes that express single-chain antibody fragments that are directed against determinants of T. cruzi (17, 18). The specificity of engineered antibody fragments for surface proteins of T. cruzi would reduce potential toxicity of transgene products to other insects or animals. Expression of several genes encoding cecropin A and recombinant antibody in a multiexpression plasmid could slow development of resistant phenotypes of T. cruzi.
Chagas disease remains a public health problem south of the U.S. border. Despite efforts by governments and overall improvement in living conditions in Central and South America, millions of people are exposed to the disease-causing parasites annually, and it remains a leading cause of cardiac and pulmonary morbidity and mortality, adding greatly to the cost of health care (19). Through the use of residual insecticides and improvement in housing standards there is a hope for eradication of this disease from human populations. Recent attempts at control have focused on elimination of vectors from domestic settings (20). These approaches show promise in regions where insect infestation is exclusively domestic in nature. When a significant sylvatic reservoir of infestation exists, these techniques alone are unsatisfactory for long-term vector control. The high cost of repeated application of insecticides and the need for long-term political commitments to such programs makes sustainability a major problem.
We here present another approach to the control of this disease. Our approach, when combined with more traditional methods of Chagas disease control, may form an effective arm of an integrated pest management program. Our approach is also applicable to many insect-borne plant, animal, and human diseases, and work on African trypanosomiasis and aphid and plant-hopper borne viral plant diseases is in progress (21).
Acknowledgments
We thank Prof. Hans Boman (University of Stockholm), Dr. Patricia de Azambuja (Fundaçao Oswaldo Cruz), Dr. Peter Mason (U.S. Department of Agriculture, Plum Island), Dr. Jean-Pierre Dujardin (ORSTOM, La Paz, Bolivia), Prof. Robert Tesh and Hilda Guzman (University of Texas Medical Branch Galveston), Dr. Robert Klein and Celia Cordon de Rosales (Medical Entomology Research Training Unit-Guatemala and Del Valle University, Guatemala City), and Brian P. Holloway and Danny L. Jue (Biotechnology Core Facility Branch, National Center for Infectious Diseases, Centers for Disease Control and Prevention) for their contributions to this project. We thank Dr. Martine Armstrong, Dr. Durland Fish, and Prof. William H. Konigsberg (Yale University) for their critical reading of this manuscript. This work was supported by the Howard Hughes Medical Institute (R.D.), the U.S. Public Health Service (AI-08614) (F.F.R.), the American Heart Association (C.B.), and the Rockefeller Foundation.
References
- 1.Georghiou, G. P. (1990) in Overview of Insecticide Resistance to Agrochemicals, American Chemical Society Symposium Series, eds. Green, M. B., LeBaron, H. M. & Moberg, W. K. (Am. Chem. Soc., Washington, DC).
- 2.Georghiou G P. Pesticide Resistance Strategies and Tactics for Management. National Research Council; Academic, New York: Board on Agriculture; 1986. pp. 11–14. [Google Scholar]
- 3.Mogi M, Teji S. Advances in Disease Vector Research. New York: Springer; 1991. pp. 47–76. [Google Scholar]
- 4.Dasch G A, Weiss E, Chang K. Bergey’s Manual of Systematic Bacteriology. Vol. 1. Baltimore: Williams & Wilkins; 1984. pp. 811–833. [Google Scholar]
- 5.Olson K E, Higgs S, Gaines P J, Powers A M, Davis B S, Kamrud K I, Carlson J O, Blair C D, Beaty B J. Science. 1996;272:884–886. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- 6.O’Brochta D A, Warren W D, Saville K J, Atkinson P W. Genetics. 1996;142:907–914. doi: 10.1093/genetics/142.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooley L R, Kelley R, Spradling A C. Science. 1988;239:1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- 8.Beard C B, Mason P W, Aksoy S, Tesh R B, Richards F F. Am J Trop Med Hyg. 1992;46:195–200. doi: 10.4269/ajtmh.1992.46.195. [DOI] [PubMed] [Google Scholar]
- 9.Boman H G, Boman I A, Andreu D, Li Z, Merrifield R B, Schlenstedt G, Zimmerman R. J Biol Chem. 1989;264:5852–5860. [PubMed] [Google Scholar]
- 10.Hultmark D, Engstrom A, Andersson K, Steiner H, Bennich H, Boman H G. EMBO J. 1983;2:571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogt Singer M E, Finnerty W R. J Bacteriol. 1988;170:638–645. doi: 10.1128/jb.170.2.638-645.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia E, DeAzambuja P, Contreras V T. In: Genes and Antigens of Parasites. A Laboratory Manual. Morel C M, editor. Rio de Janeiro: Fundacao Oswaldo Cruz; 1984. [Google Scholar]
- 13.Schägger H, Von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 14.Garcia E, DeAzambuja P. In: The Molecular Biology of Insect Disease Vectors: A Methods Manual. Crampton J, Beard C B, Louis C, editors. New York: Chapman & Hall; 1997. pp. 56–64. [Google Scholar]
- 15.Boman H G. Cell. 1991;65:205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- 16.Perlowagora-Szumlewicz A, de Carvalho Moreira C J. Mem Inst Oswaldo Cruz (Rio de Janeiro) 1994;89:603–618. doi: 10.1590/s0074-02761994000400018. [DOI] [PubMed] [Google Scholar]
- 17.Huston J S, Levinson D, Mudgett-Hunter M, Tai M, Novotny J, Margolies M N, Ridge R J, Bruccoleri R E, Haber E, Crea R, Oppermann H. Proc Natl Acad Sci USA. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia E, Azambuja P. Parasitol Today. 1991;7:240–244. doi: 10.1016/0169-4758(91)90237-i. [DOI] [PubMed] [Google Scholar]
- 19.Prata A, Macedo V. Mem Inst Oswaldo Cruz (Rio de Janeiro) 1984;79:93–96. [Google Scholar]
- 20.Gurtler R E, Petersen R M, Cecere M C. Trans Roy Soc Trop Med Hyg. 1994;88:27–30. doi: 10.1016/0035-9203(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 21.Richards F F. ASM News. 1993;59:509–514. [Google Scholar]