Abstract
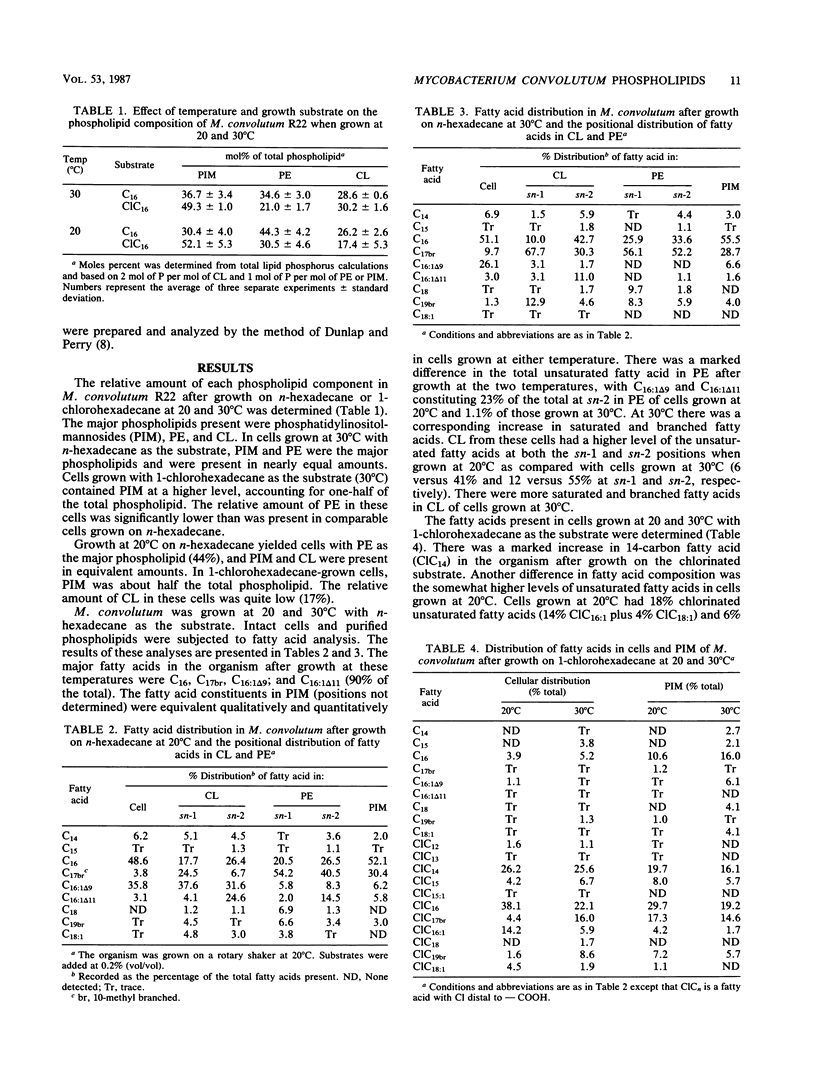
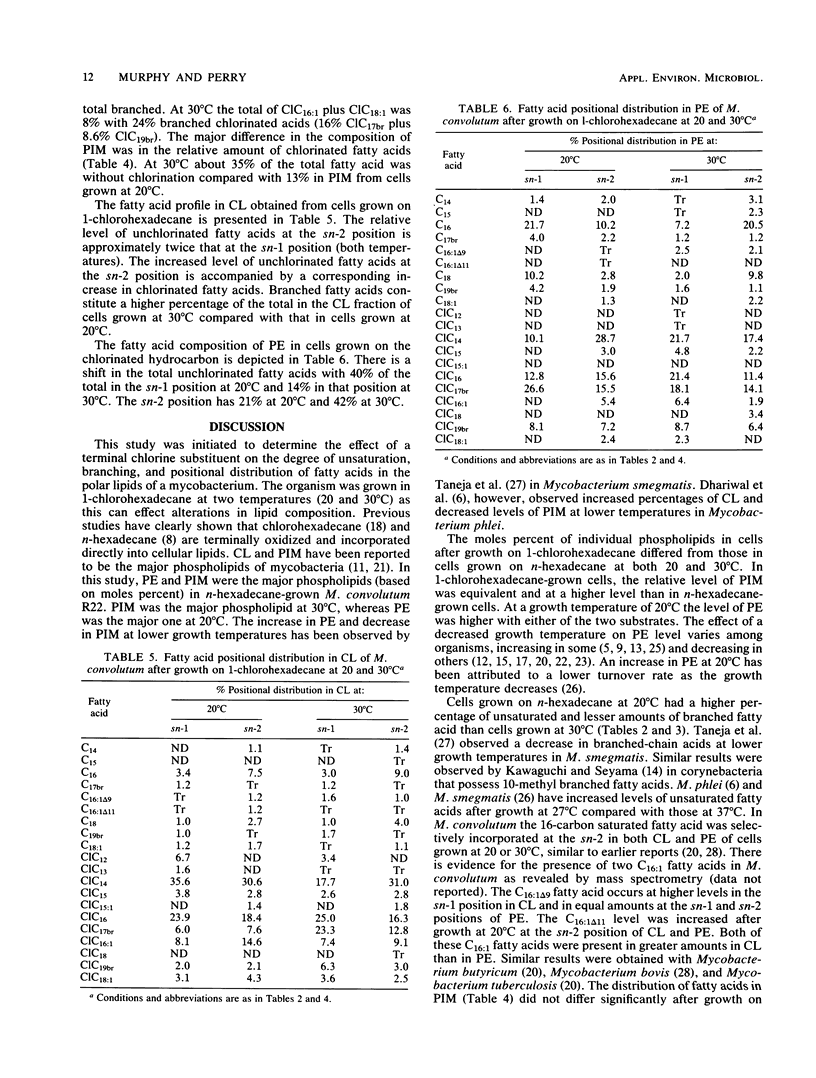
The composition of phospholipids from Mycobacterium convolutum R22 was determined after growth at two temperatures (20 and 30 degrees C) with 1-chlorohexadecane as the substrate. Comparisons were made with the phospholipids of cells grown on n-hexadecane. Phosphatidylinositolmannosides and phosphatidylethanolamine (PE) were the major phospholipids in n-hexadecane-grown cells. In 1-chlorohexadecane-grown cells, phosphatidylinositolmannosides were approximately half of the total phospholipids, with lesser amounts of PE and cardiolipin (CL). The relative level of PE was greater at 20 degrees C (versus that at 30 degrees C) after growth on either substrate. A determination was made of structure and positional distribution of constituent fatty acid in both CL and PE. The relative amount of unsaturated fatty acid was higher at 20 degrees C. There were two C16:1 fatty acids (C16:1 delta 9 and C16:1 delta 11), and these had positional preferences in both CL and PE. The positional sites of chlorinated fatty acids differed in both CL and PE at the two temperatures. The results confirm that microorganisms can specifically distribute chlorinated fatty acids into cellular phospholipids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blevins W. T., Perry J. J. Metabolism of Propane, n-Propylamine, and Propionate by Hydrocarbon-Utilizing Bacteria. J Bacteriol. 1972 Oct;112(1):513–518. doi: 10.1128/jb.112.1.513-518.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- De Siervo A. J. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J Bacteriol. 1969 Dec;100(3):1342–1349. doi: 10.1128/jb.100.3.1342-1349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. R., Perry J. J. Effect of substrate on the fatty acid composition of hydrocabon-utilizing microorganisms. J Bacteriol. 1967 Dec;94(6):1919–1923. doi: 10.1128/jb.94.6.1919-1923.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg A. D., Corner T. R. Effects of growth temperature on protoplast membrane properties in Bacillus megaterium. Can J Microbiol. 1978 Apr;24(4):386–396. doi: 10.1139/m78-065. [DOI] [PubMed] [Google Scholar]
- Goldfine H. Comparative aspects of bacterial lipids. Adv Microb Physiol. 1972;8:1–58. doi: 10.1016/s0065-2911(08)60187-3. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Mycobacterial lipids: selected topics. Bacteriol Rev. 1972 Mar;36(1):33–64. doi: 10.1128/br.36.1.33-64.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y., Kawada N., Nosoh Y. Change in chemical composition of membrane of Bacillus caldotenax after shifting the growth temperature. Arch Microbiol. 1980 Jun;126(2):103–108. doi: 10.1007/BF00511214. [DOI] [PubMed] [Google Scholar]
- Khuller G. K. Changes in phospholipid composition of Nocardia polychromogenes during temperature adaptation. Experientia. 1977 Oct 15;33(10):1277–1278. doi: 10.1007/BF01920131. [DOI] [PubMed] [Google Scholar]
- Miller K. J. Effects of temperature and sodium chloride concentration on the phospholipid and fatty acid compositions of a halotolerant Planococcus sp. J Bacteriol. 1985 Apr;162(1):263–270. doi: 10.1128/jb.162.1.263-270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. L., Perry J. J. Assimilation of chlorinated alkanes by hydrocarbon-utilizing fungi. J Bacteriol. 1984 Dec;160(3):1171–1174. doi: 10.1128/jb.160.3.1171-1174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. L., Perry J. J. Incorporation of chlorinated alkanes into fatty acids of hydrocarbon-utilizing mycobacteria. J Bacteriol. 1983 Dec;156(3):1158–1164. doi: 10.1128/jb.156.3.1158-1164.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H., Kankura T., Nojima S. Positional distribution of fatty acids in phospholipids from Mycobacteria. J Biochem. 1967 Jun;61(6):732–737. doi: 10.1093/oxfordjournals.jbchem.a128607. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan T., Murthy P. S., Gopinathan K. P. Intermediary metabolism of mycobacteria. Bacteriol Rev. 1972 Mar;36(1):65–108. doi: 10.1128/br.36.1.65-108.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raziuddin S. R. Effect of growth temperature and culture age on the lipid composition of Vibrio cholerae 569B (Inaba). J Gen Microbiol. 1976 Jun;94(2):367–372. doi: 10.1099/00221287-94-2-367. [DOI] [PubMed] [Google Scholar]
- Rilfors L., Wieslander A., Ståhl S. Lipid and protein composition of membranes of Bacillus megaterium variants in the temperature range 5 to 70 degrees C. J Bacteriol. 1978 Sep;135(3):1043–1052. doi: 10.1128/jb.135.3.1043-1052.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza K. A., Kostiw L. L., Tyson B. J. Alterations in normal fatty acid composition in a temperature-sensitive mutant of a thermophilic bacillus. Arch Microbiol. 1974 Apr 19;97(2):89–102. doi: 10.1007/BF00403049. [DOI] [PubMed] [Google Scholar]
- Taneja R., Khuller G. K. Phospholipid metabolism in Mycobacterium smegmatis ATCC 607 grown at 37 degrees and 27 C degrees C. Arch Microbiol. 1981 Mar;129(1):81–84. doi: 10.1007/BF00417185. [DOI] [PubMed] [Google Scholar]
- Taneja R., Malik U., Khuller G. K. Effect of growth temperature on the lipid composition of Mycobacterium smegmatis ATCC 607. J Gen Microbiol. 1979 Aug;113(2):413–416. doi: 10.1099/00221287-113-2-413. [DOI] [PubMed] [Google Scholar]
- Walker R. W., Barakat H., Hung J. G. The positional distribution of fatty acids in the phospholipids and triglycerides of Mycobacterium smegmatis and M. bovis BCG. Lipids. 1970 Aug;5(8):684–691. doi: 10.1007/BF02531435. [DOI] [PubMed] [Google Scholar]



