Abstract
In this study we tested the hypothesis that magainin, a peptide known to form pores in bacterial cell membranes, can increase skin permeability by disrupting stratum corneum lipid structure. We further hypothesized that magainin's enhancement requires co-administration with a surfactant chemical enhancer to increase magainin penetration into the skin. In support of these hypotheses, exposure to a known surfactant chemical enhancer, N-lauroyl sarcosine (NLS), in 50% ethanol solution increased in vitro skin permeability to fluorescein 15 fold and the combination of magainin and NLS-ethanol synergistically increased skin permeability 47 fold. In contrast, skin permeability was unaffected by exposure to magainin without co-enhancement by NLS-ethanol. Furthermore, confocal microscopy showed that magainin in the presence of NLS-ethanol penetrated deeply and extensively into stratum corneum, whereas magainin alone penetrated poorly into the skin. Additional analysis by Fourier-transform infrared spectroscopy, X-ray diffraction, and differential scanning calorimetry showed that NLS-ethanol disrupted stratum corneum lipid structure and that the combination of magainin and NLS-ethanol disrupted stratum corneum lipids even further. Altogether, these data suggest that NLS-ethanol increased magainin penetration into stratum corneum, which further increased stratum corneum lipid disruption and skin permeability. We believe this is the first study to demonstrate the use of a pore-forming peptide to increase skin permeability. This study also introduces the novel concept of using a first chemical enhancer to increase penetration of a second chemical enhancer into the skin to synergistically increase skin permeability to a model drug.
Keywords: Antimicrobial pore-forming peptide, Magainin, N-lauroylsarcosine, Stratum corneum, Surfactant chemical enhancer, Transdermal drug delivery.
1. Introduction
Transdermal delivery is an attractive method to administer drugs that avoids the pain of injection, reduces the enzymatic degradation associated with oral delivery, and facilitates sustained delivery for up to many days [1]. However, drug delivery into the skin is severely limited by the barrier properties of the stratum corneum, which is the outermost layer of skin. The stratum corneum is composed of keratin-filled, non-viable cells (corneocytes) embedded in a crystalline intercellular lipid domain. These intercellular lipids compose the continuous domain of the stratum corneum and provide its primary barrier properties. Stratum corneum lipids consist of an approximately equimolar mixture of free fatty acids, cholesterol and ceramides [2].
Numerous methods have been suggested to overcome the skin barrier for transdermal drug delivery. Chemical penetration enhancement methods have received the most attention, where addition of various chemical agents, such as fatty acids, fatty esters, alcohols, terpenes, pyrrolidones, sulfoxides, and surfactants, has been tested to increase skin permeability [3]. However, few have succeeded in delivering drugs at therapeutic rates without causing skin irritation or damage. Physical approaches, such as iontophoresis [4], electroporation [5] ultrasound [6], and microneedles [7], have also been evaluated.
In this study, we hypothesize that magainin, a peptide known to form pores in bacterial cell membranes, can increase skin permeability by disrupting stratum corneum lipid structure. A variety of pore-forming peptides are found in nature and have been the subject of intensive research due to their potential application as novel antibiotics [8]. One of the most studied is magainin, which is a 23 amino acid peptide isolated from the skin of the African clawed frog, Xenopus laevis [9]. This amphiphilic and nonhemolytic peptide has been shown to kill bacteria by perturbing the membrane function responsible for cellular osmotic balance by self-assembling in the cell membrane to form transmembrane pores [10,11]. Human clinical trials showed that a magainin derivative, Pexiganan, was developed for the intended application to infected diabetic foot ulcers [12]. Other studies have suggested that magainin might be used for contraception [13] and treatment of cancer [14].
To assess the possibility that magainin can increase skin permeability for transdermal drug delivery, our previous work showed that magainin disrupts liposome vesicles made from lipids representative of those found in human stratum corneum [15]. Encouraged by those results, in this study we have measured skin permeability after exposure to various magainin formulations and then carried out additional mechanistic analysis using Fourier-transform infrared spectroscopy, X-ray diffraction, and differential scanning calorimetry to characterize the interactions between magainin and stratum corneum lipids.
2. Materials and Methods
2.1 Skin preparation
Human cadaver skin was obtained from Emory University School of Medicine (Atlanta, GA, USA) or the National Disease Research Interchange (Philadelphia, PA, USA) with approval from the Georgia Tech IRB. After storage at −75 °C [16], whole skin was thawed in deionized water at 30 °C for 1 h. Intact epidermis was isolated from dermis using the heat separation method, in which thawed whole skin was immersed in deionized water for 2 min at 60 °C and the epidermis was then carefully peeled away from the dermis using a spatula [17]. Stratum corneum was isolated by incubating epidermis in phosphate-buffered saline (PBS) containing 0.25% trypsin (Mediatech, Herndon, VA, USA) and 0.01% gentamicin (Clonetics, Walkersville, MD, USA) at 32 °C for 24 h [18]. The isolated stratum corneum was rinsed with distilled water three times and stored on polymer-coated paper (Fisher Scientific, Waltham, MA, USA) under vacuum (KNF Neuberger, Trenton, New Jersey, USA) overnight at room temperature.
2.2 Skin permeability measurement
Before measuring skin permeability, skin was pretreated with magainin and other control formulations. Epidermis was placed in a vertical, glass Franz diffusion cell apparatus (PermeGear, Bethlehem, PA, USA) with 0.7 cm2 exposed skin surface area. The receiver chamber was filled with PBS and the donor chamber was filled with 0.3 ml of a formulation in PBS containing one or more of the following: 1 mM magainin peptide (Microchemical and Proteomics Facility, Emory University), 50% (v/v) ethanol, 2% (w/v) N-lauroyl sarcosine (NLS, 98%, Fluka, Buchs, Switzerland), cetyl trimethyl ammonium bromide, sorbitan monolaurate, oleic acid, isopropyl myristate, phenyl piperazine (all from Sigma Aldrich, St. Louis, MO, USA). After a 12-h exposure to one of these formulations at 4 °C, the Franz cell was transferred to a heater/stirrer block (PermeGear) maintained at 32 °C and stirred at 455 rpm for 3 h. The 12-h exposure was selected for experimental convenience, even though exposures between 6 − 15 h provided similar results (data not shown). This exposure was carried out at 4°C to minimize skin degradation, although preliminary experiments carried out at 25°C showed similar behavior (data not shown). The subsequent 3-h exposure was selected to produce sufficient time to re-equilibrate the skin at 32°C.
After this pretreatment, the receiver chamber was emptied and filled with fresh PBS and the donor chamber was emptied and filled with 0.3 ml of 1 mM fluorescein (Sigma Aldrich) in PBS. Every hour for 5 h, the receiver chamber was sampled by emptying it and refilling with fresh PBS. Samples were analyzed by calibrated spectrofluorimetry (Photon Technologies International, Birmingham, NJ, USA) to determine transdermal flux and permeability.
2.3 Skin imaging by multi-photon microscopy
To image fluorescein and magainin distribution in the skin, skin was pretreated for 15 h as described above, except that sulphorhodamine-tagged magainin peptide (Microchemical and Proteomics Facility, Emory University) was used. Fluorescein was delivered across the skin, as described above, for 1 h. The skin sample was then removed from the Franz cell and placed on a glass coverslip. Skin imaging was carried out at room temperature using a multi-photon microscope (Zeiss LSM/NLO 510, Oberkochen, Germany) with a water immersion lens of 20× magnification and an oil-immersion lens of 40× magnification to collect “z-stack” optical slices at a series of depths into the epidermis.
2.4 Imaging of histological skin sections
To image microscale stratum corneum organization, a 2 cm × 2 cm piece of full-thickness skin was pretreated as described above and then cut into 4 mm × 7 mm pieces and placed in a cryoblock with optimal cutting temperature compound (Tissue-Tek, Sakura Finetek, Torrance, CA, USA). After freezing in liquid nitrogen, skin was sectioned on a cryostat (Cryo-star HM 560MV, Microm, Waldorf, Germany) at 40-μm thickness and placed on a glass slide.
To help imaging the stratum corneum, the skin sections were swelled by applying a drop of half-strength Sorensen-Walbum buffer (0.1 N sodium hydroxide solution and 0.1 N glycine solution; Sigma Aldrich) to the tissue for 15 min [19]. Then, a stock solution containing 0.05% (w/v) Nile Red (Molecular Probes, Eugene, OR, USA) in acetone was diluted to 2.5 μg/ml with 75:25 (v/v) glycerol:water and vigorously mixed on a vortexer. A drop of the resulting Nile Red solution was applied on each sample, which was then sealed beneath a cover slip using transparent nail polish (Revlon, New York, USA) [19] and imaged by a multi-photon microscope (Zeiss LSM/NLO 510).
2.5 Fourier-transform infrared spectroscopy
To analyze changes in skin using Fourier-transform infrared (FTIR) spectroscopy, several pieces of stratum corneum were each soaked in various chemical enhancer formulations for 24 h at 4 °C and then washed with PBS solution. The stratum corneum samples were placed in a Magma-IR 560 FT-IR spectrometer with OMNIC professional software (Nicolet, Thermo Electron Corporation, Waltham, MA, USA) and measured at 25 °C. All spectra represented the average of 64 scans obtained with a resolution of 4 cm−1 over the range of 4,000 − 1,000 cm−1.
2.6 X-ray diffraction
Stratum corneum samples were pretreated with enhancer formulations as described above, desiccated under vacuum, and then hydrated for 10 min before examination. The wide-angle X-ray diffraction (PANalytical, Almelo, Netherlands) was carried out at 40 mA and 45 kV and small-angle X-ray diffraction (Rigaku DMAX 2500, Tokyo, Japan) was carried out at 300 mA and 50 kV.
2.7 Differential scanning calorimetry
Several pieces of stratum corneum were each soaked in various chemical enhancer formulations for 24 h at 4 °C and then washed with PBS. The stratum corneum samples were desiccated under vacuum for 12 h and then hermetically sealed within an aluminum holder (PerkinEelmer, Wellesley, MA, USA). Desiccation has been shown to sharpen DSC thermal transitions without altering the location of stratum corneum lipid peaks.[20] Thermal analysis was carried out by heating samples from 0 °C to 120 °C at a rate of 10 °C/min using a DSCQ100 differential scanning calorimeter fitted with a refrigerated cooling system (TA Instruments, New Castle, DE, USA).
2.8. Statistical Analysis
Skin permeability to fluorescein, FTIR spectroscopy, and DSC were measured using at least three replicate skin samples at each condition, from which the mean and standard error of the mean were calculated. A two-tailed Student's t-test was performed when comparing two different conditions. When comparing three or more conditions, a one-way analysis of variance (ANOVA) was performed.
3. Results
3.1 Effect of magainin formulations on skin permeability
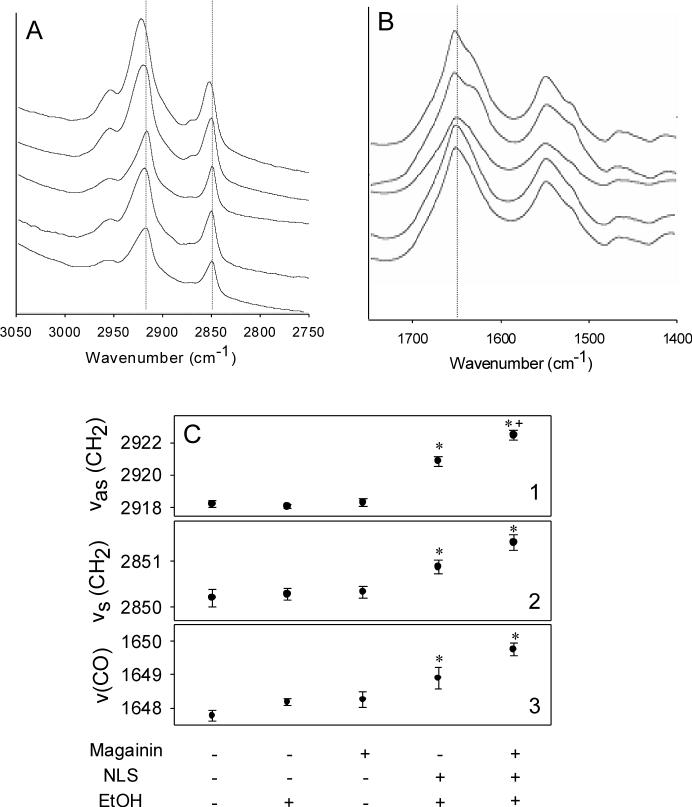
Our previous results showed that incubation with magainin peptide could disrupt vesicles made from lipids representative of those found in human stratum corneum [15]. This suggested that magainin could disrupt stratum corneum lipids in the skin and thereby increase skin permeability. To test this idea, we exposed human skin to a magainin solution, but there was no change in skin permeability to a model compound, fluorescein (Fig. 1, Student's t-test, p=0.35). To explain this result, we hypothesized that magainin has the ability to insert into a single bilayer, such as that found in a bacterial cell or a liposome vesicle, but may not have a mechanism to cross over additional bilayers, especially given magainin's large size (2494 Da). Because there are close to 100 multilamellar lipid bilayers in a cross section of stratum corneum, the ability to cross multiple bilayers is essential to increase skin permeability.
Figure 1.
Effect of magainin formulations on skin permeability. (A) Cumulative fluorescein delivered across human cadaver skin pre-treated with (•) no treatment, (▼) magainin peptide in PBS, (○) 50% ethanol, (▽) NLS in 50% ethanol and (■) NLS and magainin peptide in 50% ethanol. (B) Skin permeability values determined from steady-state transdermal fluxes shown in part (A). Data represent averages of n≥3 samples with standard error of the mean. The * symbol identifies permeabilities significantly greater than untreated skin (Student's t-test, p<0.05). The + symbol identifies permeabilities after magainin exposure significantly greater than without magainin exposure (Student's t-test, p<0.05).
To address this problem, we added a well-known anionic surfactant enhancer – N-lauroyl sarcosine (NLS) – in 50% ethanol solution to facilitate magainin penetration throughout the stratum corneum. We used this formulation, because our previous study showed that NLS in 50% ethanol is a good skin permeation enhancer [21]. As shown in Fig. 1, this magainin-NLS-ethanol combination yielded a 47-fold increase in skin permeability(Student's t-test, p<0.01), which shows that magainin can increase skin permeability.
Moreover, the enhancement by magainin-NLS-ethanol was significantly greater than the 15-fold increase caused by NLS in ethanol (without magainin) (Student's t-test, p<0.01) or the 1.3 fold increase caused by ethanol (without magainin or NLS) (Student's t-test, p<0.01). Because the enhancement of the magainin-NLS-ethanol combination is three-fold greater than the sum of the enhancements by the individual components, this synergy suggests an interaction between magainin and NLS-ethanol and is consistent with the hypothesis that NLS-ethanol increased magainin penetration into skin, which in turn allowed magainin to further disrupt lipid bilayer structures in stratum corneum.
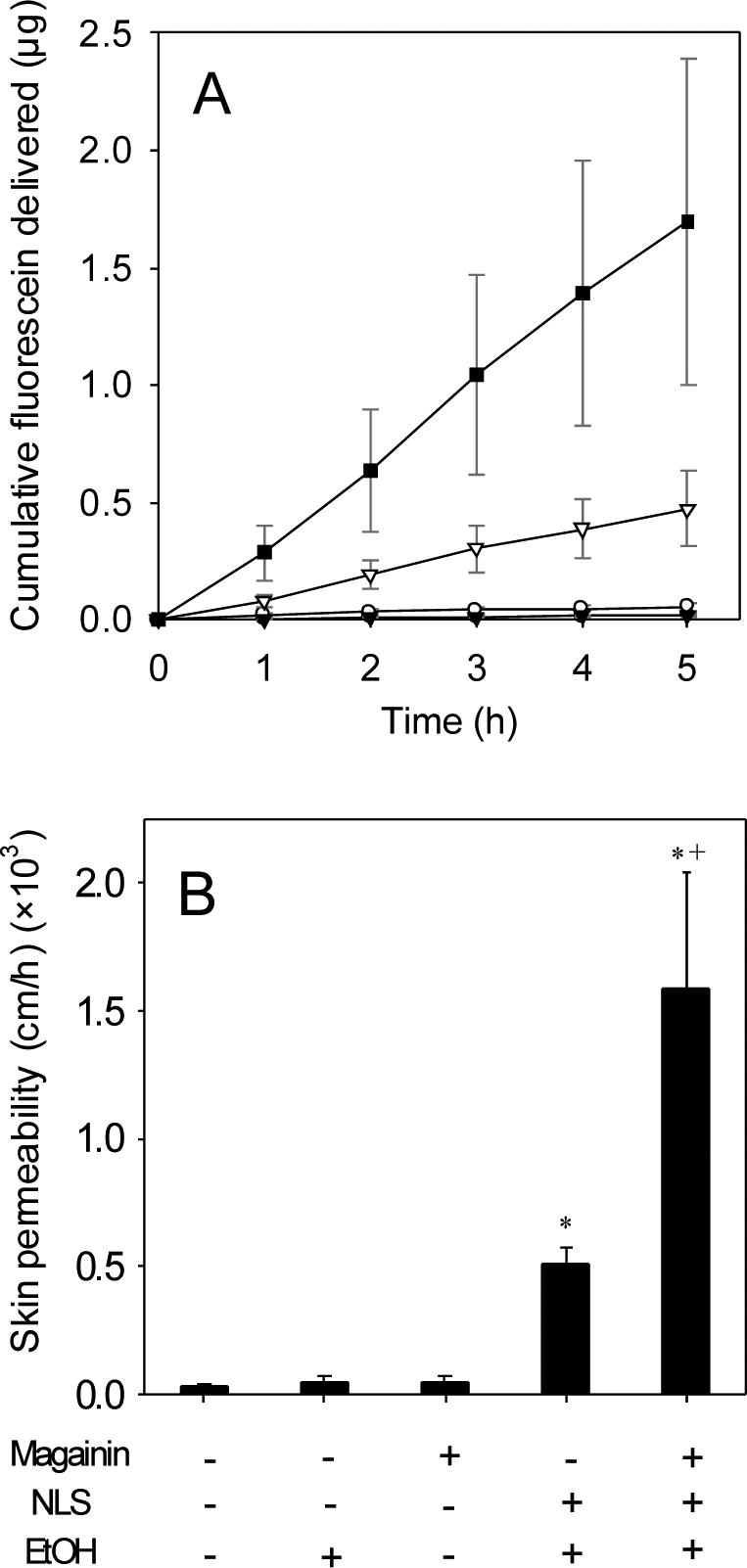
To determine if other chemical enhancer formulations might similarly act synergistically with magainin, we compared NLS to five other enhancers from different chemical classes, including a cationic surfactant (cetyl trimethyl ammonium bromide), nonionic surfactant (sorbitan monolaurate), fatty acid (oleic acid), fatty ester (isopropyl myristate), and Azone-like compound (phenyl piperazine). As shown in Fig. 2, only NLS and the cationic surfactant directly increased skin permeability to fluorescein (conditions B and C, Student's t-test, p<0.01) and only NLS showed a synergistic increase in skin permeability with magainin (condition B, Student's t-test, p=0.046). The fact that magainin carries a positive charge and NLS carries a negative charge suggests that the ionic attraction between these compounds may be important to their action.
Figure 2.
Effect of chemical enhancers on skin permeability and interaction with magainin. Skin permeability to fluorescein after pre-treatment with an enhancer alone (black bars) or in combination with magainin (white bars) for different enhancers: (A) no chemical enhancer, (B) N-lauroyl sarcosine, (C) cetyl trimethyl ammonium bromide, (D) sorbitan monolaurate, (E) oleic acid, (F) isopropyl myristate and (G) phenyl piperazine, all in 50% ethanol. Data represent averages of n≥3 samples with standard error of the mean. The * symbol identifies permeabilities significantly greater than untreated skin (Student's t-test, p<0.05). The + symbol identifies permeabilities after magainin exposure significantly greater than without magainin exposure (Student's t-test, p<0.05).
3.2 Imaging magainin and fluorescein penetration into skin
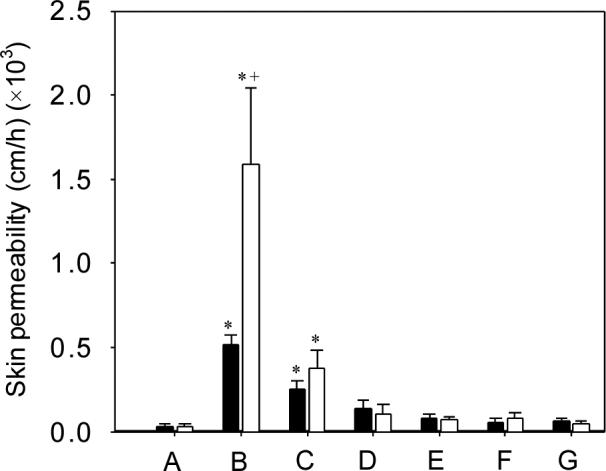
To more directly test the hypothesis that NLS-ethanol increases magainin penetration into skin, we imaged the skin by multi-photon microscopy after delivery of sulforhodamine-labeled magainin with and without NLS-ethanol enhancement. As shown in Fig. 3A, very little magainin (red fluorescence) was able to enter the skin without enhancement. In contrast, Fig. 3B shows that in the presence of NLS-ethanol, magainin was able to penetrate throughout the 10 − 15 μm layer of stratum corneum.
Figure 3.
Penetration of sulforhodamine-tagged magainin peptide into human epidermis imaged by multi-photon microscopy. Magainin formulated (A) without NLS and (B) with NLS, both in 50% ethanol. Optical sections taken at 5 μm increments starting at the stratum corneum surface on the left and proceeding deeper on the right. Scale bar is 100 μm.
Fig. 4 shows complementary data that simultaneously image magainin (red fluorescence) and fluorescein (green fluorescence) transport into the skin. In the absence of NLS-ethanol and magainin, there is little fluorescein delivery into the skin (Fig. 4A). The addition of NLS-ethanol significantly increased fluorescein delivery (Fig. 4B), in agreement with skin permeability data in Fig. 1. Finally, the combination of NLS-ethanol and magainin increased fluorescein delivery even more and was associated with significant magainin penetration into the stratum corneum (Fig. 4C). It is interesting to note that there is significant co-localization of magainin and fluorescein (indicated by yellow in Fig. 4C), which is consistent with magainin-mediated disruption of the stratum corneum corresponding to pathways for increased fluorescein transport. It is also worth noting that magainin preferentially localized within the stratum corneum, which is consistent with its expected mechanism of preferentially inserting into stratum corneum lipid bilayers, whereas fluorescein penetrated deeply into the skin.
Figure 4.
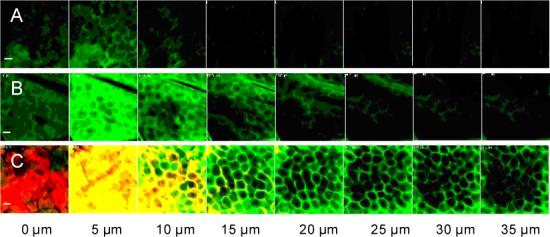
Penetration of fluorescein and sulforhodamine-tagged magainin peptide into human epidermis imaged by multi-photon microscopy. Fluorescein formulated with (A) PBS, (B) with NLS in 50% ethanol and (C) with NLS and magainin in 50% ethanol. Green corresponds to fluorescein, red corresponds to sulforhodamine-tagged magainin and yellow corresponds to co-localization of fluorescein and magainin. Optical sections take at 5 μm increments starting at the stratum corneum surface on the left and proceeding deeper on the right. Scale bar is 100 μm.
These observations provide direct evidence that increased magainin penetration into the stratum corneum is associated with increased skin permeability and that NLS-ethanol facilitates that increased magainin penetration. This insight not only helps elucidate the mechanism of skin permeability enhancement, but also introduces the novel concept of using a first chemical enhancer to increase delivery of second chemical enhancer into the skin.
3.3 Fourier-transform infrared spectroscopy
Because magainin and NLS-ethanol are hypothesized to disrupt stratum corneum lipid structure, we characterized stratum corneum samples exposed to different formulations using Fourier-transform infrared (FTIR) spectroscopy. To identify alterations in lipid order, we measured changes in carbon-hydrogen stretching frequencies near 2850 and 2920 cm−1, which are due to symmetric [vs(CH2)] and asymmetric [vas(CH2)] C-H stretching in stratum corneum lipid molecules, respectively (Fig. 5A) [22]. To identify alteration in protein structure, we also measured changes in the carbon-oxygen stretching frequency near 1650 cm−1 [v(CO)], which is associated with transitions from α-helix to β-sheet structure of stratum corneum keratin molecules (Fig. 5B) [23].
Figure 5.
Fourier-transform infrared spectroscopy analysis of human stratum corneum treated with different formulations. Spectra of wavenumbers characteristic of (A) C-H stretching in lipids and (B) C-O stretching in proteins treated with (from bottom to top): PBS, magainin in PBS, 50% ethanol, NLS in 50% ethanol, NLS and magainin in 50% ethanol. Dashed lines indicated peaks of interest. Graphs are representative of n≥3 replicate samples. (C) Change of (1) CH2 asymmetric stretching frequency, (2) CH2 symmetric stretching frequency and (3) CO stretching frequency, determined from graphs in (A) and (B). Data represent averages of n≥3 samples with standard error of the mean. The * symbol identifies wavenumbers significantly greater than untreated skin (Student's t-test, p<0.05). The + symbol identifies wavenumbers after exposure to NLS and magainin that are significantly greater than exposure to just NLS (Student's t-test, p<0.05).
As summarized in Fig. 5C, stratum corneum treated with magainin alone or 50% ethanol alone did not cause significant changes in any of the three characteristic stretching frequencies (Student's t-test, p>0.05). In contrast, treatment with NLS-ethanol caused an increase in νas(CH2) of 2.6 cm−1 and νs(CH2) of 0.85 cm−1 relative to untreated skin (Student's t-test, p<0.05). Addition of magainin peptide raised these stretching frequency values even higher to increases in νas(CH2) of 4.3 cm−1 and νs(CH2) of 1.2 cm−1. These increases in the presence of magainin are significantly different from untreated skin (Student's t-test, p<0.01) and significantly different from skin treated only with NLS-ethanol for νas(CH2) (Student's t-test, p<0.05), but not for νs(CH2) (Students t-test, p>0.05). Although these wavenumber shifts are relatively small, they are consistent in magnitude with previous studies of chemical enhancers.[24] Altogether, these data indicate that NLS-ethanol increased stratum corneum lipid chain disorder and fluidity and that addition of magainin caused a further increase in this disorder.
Protein structure was also affected by NLS-ethanol and magainin. Treatment with NLS-ethanol caused an increase in ν(CO) of 1.1 cm−1 relative to untreated skin (Student's t-test, p=0.028). Addition of magainin peptide raised ν(CO) even higher to an increase of 2 cm−1, which is significantly greater than untreated skin (Student's t-test, p=0.002) but not significantly different from skin treated only with NLS-ethanol (Student's t-test, p=0.091). These data indicate that NLS-ethanol and magainin also have effects on stratum corneum proteins, possibly promoting a transformation from α-helix to β-sheet structure.
3.4 X-ray diffraction
To further elucidate effects on stratum corneum structure, we used wide-angle and small-angle X-ray diffraction (XRD) to provide a direct measure of changes in lipid bilayer packing. Wide-angle XRD on untreated skin revealed a characteristic peak at 16.7 Å caused by scattering of crystalline cholesterol [25], which is a primary component of stratum corneum lipids (Fig. 6A). Treatment with NLS-ethanol reduced this peak and addition of magainin reduced it still farther. This suggests that both NLS-ethanol and magainin reduced cholesterol crystallinity, which reduces lipid bilayer order .[26]
Figure 6.
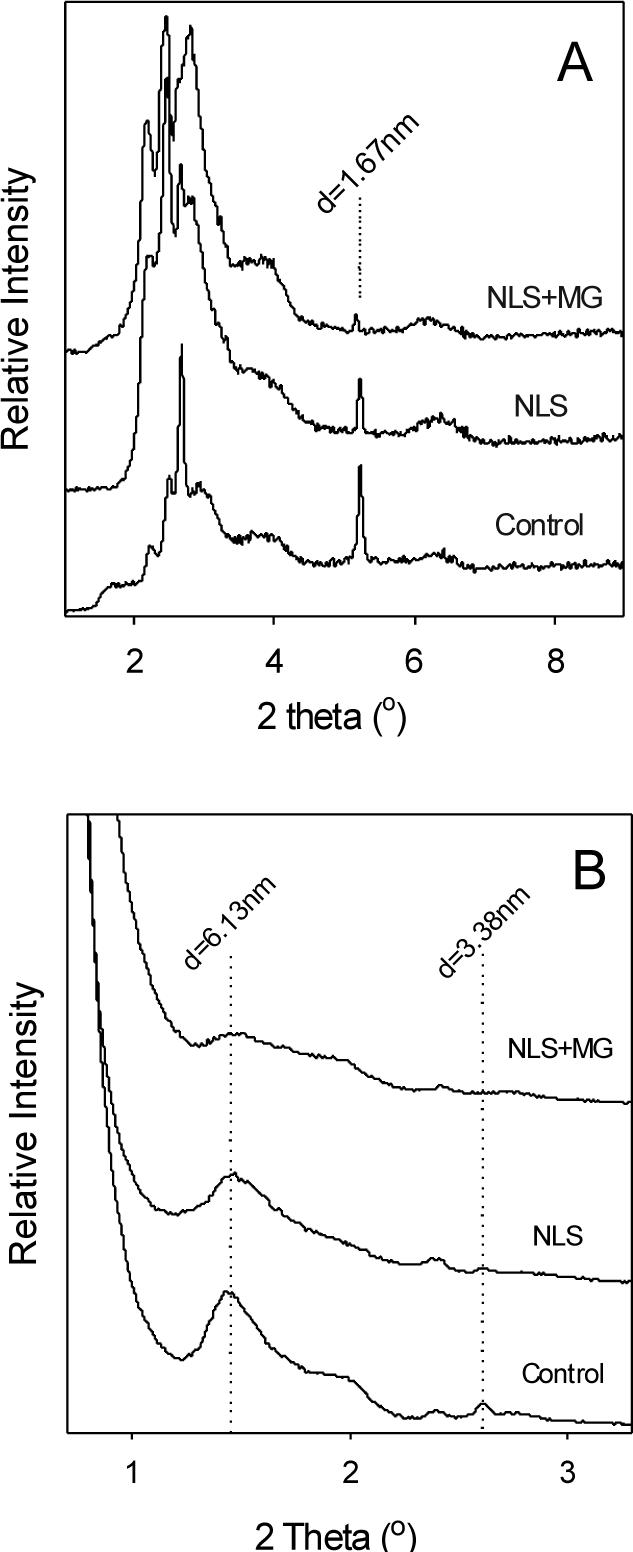
X-ray scattering analysis of human stratum corneum treated with different formulations. (A) Wide-angle and (B) small-angle X-ray scattergrams of stratum corneum treated with PBS (control), NLS in 50% ethanol or NLS and magainin in 50% ethanol. Dashed lines indicate peaks of interest. Graphs are representative of n≥3 replicate samples.
Small-angle XRD on untreated skin revealed a number of characteristic peaks, notably corresponding to ceramides (d = 6.13 nm) and crystalline cholesterol (d = 3.38 nm) [27]. Treatment with NLS-ethanol reduced both the ceramide and crystalline cholesterol peaks and addition of magainin reduced them still farther. This further suggests that both NLS-ethanol and magainin disrupt the order of the dominant components of stratum corneum lipid bilayer structures.
3.5 Differential scanning calorimetry
Differential scanning calorimetry (DSC) provides another method to probe changes in stratum corneum structure, based on its thermal properties. DSC analysis of untreated dry human stratum corneum showed two major thermal transitions, T1 and T2, at approximately 75 and 90 °C, respectively (Fig. 7). T1 is assigned to lipid structure transformation from lamellar to the disordered, and T2 is assigned to a protein-associated lipid transition from gel to liquid form [28]. Other previously reported transition peaks at 35−40 °C and 105−120 °C were not seen here, in part because the stratum corneum samples were desiccated after treatment [29,30].
Figure 7.
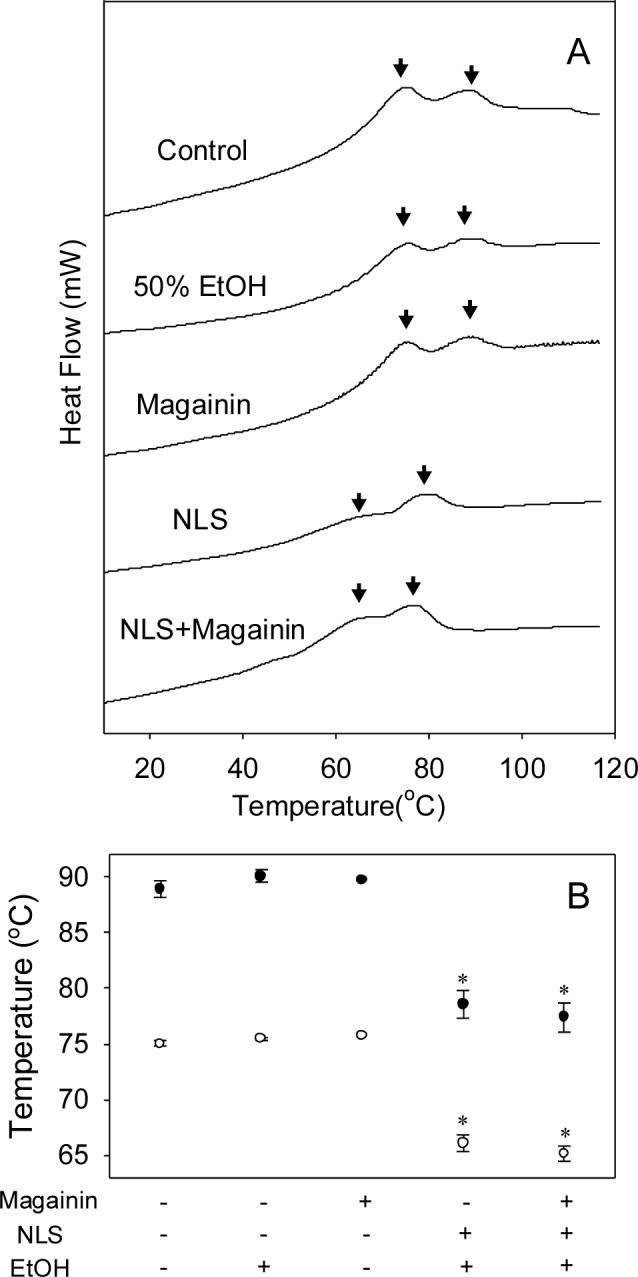
Differential scanning calorimetry (DSC) analysis of human stratum corneum treated with different formulations. (A) Thermograms of stratum corneum treated with (from top to bottom): PBS (control), magainin in PBS, 50% ethanol, NLS in 50% ethanol, NLS and magainin in 50% ethanol. Arrows indicate peaks of interest. Graphs are representative of n≥3 replicate samples. (B) Changes of two characteristic transition midpoint temperatures (T1: ●, T2: ○) determined from graph in (A). Data represent averages of n≥3 samples with standard error of the mean. The * symbol identifies temperatures significantly smaller than untreated skin (Student's t-test, p<0.05).
As summarized in Fig. 7B, stratum corneum treated with magainin alone or 50% ethanol alone did not cause significant changes in either of the major thermal transitions (Student's t-test, p>0.1). In contrast, after treatment with NLS-ethanol, the first and second transition temperatures were decreased by 9 and 10.5 °C, respectively, relative to untreated skin (Student's t-test, p<0.01). Addition of magainin further dropped these transition temperatures to decreases of 10 and 11.5 °C, respectively (Student's t-test, p<0.01), but these changes were not significantly different from skin treated only with NLS-ethanol (Student's t-test, p>0.1). Altogether, the decreased lipid transition temperatures indicate that NLS-ethanol disordered stratum corneum lipids, but that addition of magainin did not further disorder lipids at detectable levels. The effects of magainin may not have been evident through DSC analysis because DSC measures bulk properties that are relatively insensitive to localized changes. Unlike NLS-ethanol, which can fluidize throughout the stratum corneum lipids, magainin's mechanism of action is expected to be in the form of transmembrane pore structures localized on the nanometer scale.
3.6 Stratum corneum histology
As a final assessment of changes to stratum corneum structure, skin was cryo-sectioned, stained and imaged histologically after exposure to different formulations. As displayed in Fig. 8A, untreated stratum corneum showed intact, densely packed structure. In contrast, stratum corneum treated with NLS-ethanol alone (Fig. 8B) or with magainin peptide (Fig. 8C) showed disruption and expansion of the stratum corneum layers consistent with a loss of lipid structural order. Differences between stratum corneum treated with or without magainin peptide were, once again, not evident, perhaps because localized, nanometer-scale pore structures believed to be formed by magainins are not visible by optical microscopy.
Figure 8.
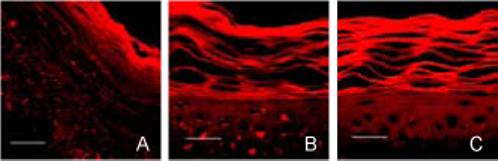
Changes in human stratum corneum architecture imaged by multi-photon microscopy of histological cross-sections. Epidermis was treated with (A) PBS, (B) NLS in 50% ethanol and (C) NLS and magainin in 50% ethanol. To facilitate imaging, stratum corneum was swelled by incubation in Sorensen-Walbum buffer and stained with Nile Red. Scale bar is 20 μm.
4. Discussion
4.1 Magainin can increase skin permeability by disrupting stratum corneum lipid structure
This study investigated the application of a pore-forming peptide, magainin, as a novel transdermal transport enhancer. Toward this end, we tested the hypothesis that magainin, a peptide known to form pores in bacterial cell membranes, can increase skin permeability by disrupting stratum corneum lipid structure. In support of this hypothesis, a magainin-based formulation was shown to increase skin permeability to fluorescein by 47 fold (Fig. 1). The mechanism of disrupting stratum corneum lipid structure was supported by multi-photon microscopy imaging, shifted FTIR peak position related to lipid and protein stretching frequencies, and reduced X-ray diffraction peak intensities related to lipid structure.
Multi-photon microscopy showed significant magainin penetration into the stratum corneum, but not into the viable epidermis (Figs. 3 and 4). This indicates that the site of action is located in the stratum corneum. Although magainin appears to be localized both intracellularly and extracellularly in the stratum corneum, fluorescein appears to be located primarily extracellularly (i.e., within the extracellular lipids). This indicates that the site of magainin action to increase skin permeability was located in the extracellular lipids.
FTIR spectroscopy showed a disruption of stratum corneum lipid and protein structure induced by magainin (Fig. 5). Moreover, for the various formulations used, the degree of structural disruption measured by FTIR correlated with the increase in skin permeability measured by fluorescein delivery, which suggests a mechanistic relationship. Disruption of lipid acyl chain order was indicated by an increase of C-H stretching absorbances to higher wavenumbers [22]. This disruption could be from a homogeneous fluidization of the lipid domain and, possibly, from heterogeneous defects in lipid structure due to discrete pores stabilized by magainins and due to pooling of NLS and magainin in discrete domains among the lipids. Protein order was also disrupted, as indicated by an increase in C-O stretching consistent with conformational transformation of keratin from α-helix to β-sheet structure [23]. This conformational change of keratin may be a secondary effect of the disruption of stratum corneum lipid structure [31].
X-ray scattering analysis also indicated a disruption of stratum corneum lipid structure induced by magainin (Fig. 6). Again, there was a dose-response effect, where formulations that caused greater structural disruption corresponded to increased skin permeability. Wide-angle and small-angle XRD showed reduced peak intensity associated with crystalline cholesterol. Cholesterol is the second most abundant lipid in the stratum corneum and its role is recognized as providing plasticity in the gel-phase bilayer [32]. On a related note, it has been observed that changes in cholesterol metabolism in the epidermis leads to a disturbed barrier function of skin [33]. Our formulations containing NLS-ethanol and magainin are hypothesized to increase skin permeability in part by removing or reducing the cholesterol in stratum corneum. Previous work has shown that other anionic surfactants, sodium lauryl sulfate and lauroyl isothionate, extract cholesterol from stratum corneum cell membranes [34]. Small-angle XRD also showed reduced peak intensity associated with ceramides, which is also one of the most abundant lipids in stratum corneum.
DSC analysis showed changes in the thermal profile of stratum corneum after treatment with NLS-ethanol that was indicative of increased lipid fluidity. However, further changes in the thermal profile due to addition of magainin were not statistically significant.
4.2 Magainin's enhancement requires co-administration with a surfactant chemical enhancer to increase magainin penetration into the skin
Although magainin was previously shown to disrupt liposome vesicles made of lipids representative of stratum corneum [15], magainin without a chemical enhancer had no effect on skin permeability in this study (Fig. 1). This could be explained by the ability of magainin to inserted into and disrupt an individual lipid bilayer, but an inability to cross over multiple bilayers, such as the multilamellar stacks of lipids found in stratum corneum. Guided by this, we hypothesized that magainin's enhancement requires co-administration with a surfactant chemical enhancer to increase magainin penetration into the skin.
Consistent with this hypothesis, co-administration of magainin and an anionic surfactant, NLS, in a 50% ethanol formulation increased skin permeability 47 fold. In contrast, NLS-ethanol without magainin increase skin permeability just 15 fold (Fig. 1). This synergistic effect indicated an interaction between NLS and magainin. Multi-photon microscopy supported this, by showing that without NLS-ethanol, little magainin penetration into the skin occurred, but co-administration of magainin and NLS-ethanol led to extensive magainin penetration throughout the stratum corneum (Fig. 3). Comparison to fluorescein transport data (Figs. 1 and 4) showed that increased magainin penetration into stratum corneum correlated with increased skin permeability.
In addition to NLS, five other kinds of chemical enhancers were evaluated, but only the anionic surfactant NLS was found to act synergistically with magainin. This could be because of an ionic attraction between negatively charged NLS and positively charged magainin that facilitates penetration and interaction with the stratum corneum. It also might be explained based on an interaction between NLS and skin that makes stratum corneum especially permeable and thereby, in a nonspecific way, increases magainin penetration into stratum corneum. This latter argument is supported by the observation that, without magainin, NLS increased skin permeability to fluorescein better than the other five chemical enhancers.
4. Conclusions
This study showed that a formulation containing a pore-forming peptide, magainin, and NLS in 50% ethanol synergistically increased skin permeability by 47 fold. Analysis by multi-photon microscopy, FTIR, XRD, and DSC supported the hypothesis that magainin can increase skin permeability by disrupting stratum corneum lipid structure, especially ceramides and cholesterol. Additional analysis supported the hypothesis that magainin's enhancement requires co-administration with a surfactant chemical enhancer to increase magainin penetration into the skin
Overall, we conclude that the combination of magainin and NLS-ethanol synergistically increases skin permeability, because NLS-ethanol increased magainin penetration into stratum corneum, which further increased stratum corneum lipid disruption and skin permeability. We believe this is the first study to use a pore-forming peptide as a skin penetration enhancer and the first study to use one chemical enhancer to increase penetration of another chemical enhancer into the skin.
Acknowledgements
We thank Han-Gi Chae, Jin-Ho Choy, Seong-Ho Park, and Won-Tae Oh for helpful technical assistance. Mark Prausnitz is the Emerson-Lewis Faculty Fellow. This work was carried out in the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug. Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 2.Wertz PW. Lipids and barrier function of the skin. Acta Derm. 2000:7–11. doi: 10.1080/000155500750042790. [DOI] [PubMed] [Google Scholar]
- 3.Williams AC, Barry BW. Penetration enhancers. Adv. Drug. Deliv. Rev. 2004;56:603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv. Drug. Deliv. Rev. 2004;56:619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Denet AR, Vanbever R, Preat V. Skin electroporation for transdermal and topical delivery. Adv. Drug. Deliv. Rev. 2004;56:659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Mitragotri S, Kost J. Low-frequency sonophoresis - A review. Adv. Drug. Deliv. Rev. 2004;56:589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Prausnitz MR. Microneedles for transdermal drug delivery. Adv. Drug. Deliv. Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophy. Acta. 1999;1462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 9.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin - isolation, characterization of 2 active forms, and partial c-DNA sequence of a precursor. Proc. Natl. Acad. Sci. U. S. A. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang HW, Chen FY, Lee MT. Molecular mechanism of peptide-induced pores in membranes. Phy. Rev. Lett. 2004;92:198304. doi: 10.1103/PhysRevLett.92.198304. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzaki K, Sugishita K, Harada M, Fujii N, Miyajima K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophy. Acta. 1997;1327:119–130. doi: 10.1016/s0005-2736(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 12.Nelson EA, O'Meara S, Golder S, Dalton J, Craig D, Iglesias C. Systematic review of antimicrobial treatments for diabetic foot ulcers,. Diabet. Med. 2006;23:348–359. doi: 10.1111/j.1464-5491.2006.01785.x. [DOI] [PubMed] [Google Scholar]
- 13.Clara A, Manjramkar DD, Reddy VK. Preclinical evaluation of magainin-A as a contraceptive antimicrobial agent. Fert. Steril. 2004;81:1357–1365. doi: 10.1016/j.fertnstert.2003.09.073. [DOI] [PubMed] [Google Scholar]
- 14.Ohsaki Y, Gazdar AF, Chen HC, Johnson BE. Antitumor-activity of magainin analogs against human lung-cancer cell-lines. Cancer Res. 1992;52:3534–3538. [PubMed] [Google Scholar]
- 15.Kaushik S, Krishnan A, Prausnitz MR, Ludovice PJ. Magainin-mediated disruption of stratum corneum lipid vesicles. Pharm. Res. 2001;18:894–896. doi: 10.1023/a:1011013218494. [DOI] [PubMed] [Google Scholar]
- 16.Harrison SM, Barry BW, Dugard PH. Effects of freezing on human-skin permeability. J. Pharma. Pharmacol. 1984;36:261–262. doi: 10.1111/j.2042-7158.1984.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 17.Scheuplein R. Mechanism of percutaneous adsorption. I. Routes of penetration and influence of solubility. J. Invest. Dermatol. 1965;45:334–346. doi: 10.1038/jid.1965.140. [DOI] [PubMed] [Google Scholar]
- 18.Kuriharabergstrom T, Knutson K, Denoble LJ, Goates CY. Percutaneous-absorption enhancement of an ionic molecule by ethanol water-systems in human skin. Pharm. Res. 1990;7:762–766. doi: 10.1023/a:1015879925147. [DOI] [PubMed] [Google Scholar]
- 19.Talreja PS, Kleene NK, Pickens WL, Wang TF, Kasting GB. Visualization of the lipid barrier and measurement of lipid pathlength in human stratum corneum. AAPS. PharmSci. 2001;3:1–9. doi: 10.1208/ps030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman M, Barry BW. Differential scanning calorimetry of human stratum corneum: Effects of penetration enhancers Azone and dimethyl sulphoxide. Anal. Proceed. 1986;23:397–398. [Google Scholar]
- 21.Kim Y, Ludovice PJ, Prausnitz MR. Synergistic enhancement of skin permeability by N-lauroylsarcosine and ethanol. J. Pharm. Sci. doi: 10.1016/j.ijpharm.2007.10.031. submitted. [DOI] [PubMed] [Google Scholar]
- 22.Casal HL, Mantsch HH. Polymorphic phase-behavior of phospholipid-membranes studied by infrared-spectroscopy. Biochim. Biophy. Acta. 1984;779:381–401. doi: 10.1016/0304-4157(84)90017-0. [DOI] [PubMed] [Google Scholar]
- 23.Goates CY, Knutson K. Enhanced permeation of polar compounds through human epidermis. 1. Permeability and membrane structural-changes in the presence of short-chain alcohols. Biochim. Biophy. Acta. 1994;1195:169–179. doi: 10.1016/0005-2736(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 24.Potts RO, Golden GM, Francoeur ML, Mak VHW, Guy RH. Mechanism and enhancement of solute transport across the stratum-corneum. J. Control. Release. 1991;15:249–260. [Google Scholar]
- 25.Lopez O, Cocera M, Campos L, de la Maza A, Coderch L, Parra JL. Use of wide and small angle X-ray diffraction to study the modifications in the stratum corneum induced by octyl glucoside. Colloids. Surf. A. 2000;162:123–130. [Google Scholar]
- 26.Kitagawa S, Sawada M, Hirata H. Fluorescence analysis with diphenylhexatriene and its ionic derivatives of the fluidity of liposomes constituted from stratum-corneum lipids - Contribution of each lipid component and effects of long-chain unsaturated fatty-acids. Int. J. Pharm. 1993;98:203–208. [Google Scholar]
- 27.Schreiner V, Gooris GS, Pfeiffer S, Lanzendorfer G, Wenck H, Diembeck W, Proksch E, Bouwstra J. Barrier characteristics of different human skin types investigated with X-ray diffraction, lipid analysis, and electron microscopy imaging. J. Invest. Dermatol. 2000;114:654–660. doi: 10.1046/j.1523-1747.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 28.Tanojo H, Bouwstra JA, Junginger HE, Bodde HE. Thermal analysis studies on human skin and skin barrier modulation by fatty acids and propylene glycol. J. Therm. Anal. Calorim. 1999;57:313–322. [Google Scholar]
- 29.Golden GM, Guzek DB, Harris RR, Mckie JE, Potts RO. Lipid thermotropic transitions in human stratum-corneum. J. Invest. Dermatol. 1986;86:255–259. doi: 10.1111/1523-1747.ep12285373. [DOI] [PubMed] [Google Scholar]
- 30.Leopold CS, Lippold BC. An attempt to clarify the mechanism of the penetration enhancing effects of lipophilic vehicles with differential scanning calorimetry (DSC) J. Pharm. Pharmacol. 1995;47:276–281. doi: 10.1111/j.2042-7158.1995.tb05795.x. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi Y, Yasukawa H, Yamaoka Y, Kato Y, Morimoto Y, Fukumori Y, Fukuda T. Effects of fatty-acids, fatty amines and propylene-glycol on rat stratum-corneum lipids and proteins in vitro measured by Fourier-transform infrared attenuated total reflection (FT-IR/ATR) spectroscopy. Chem. Pharm. Bull. 1992;40:1887–1892. doi: 10.1248/cpb.40.1887. [DOI] [PubMed] [Google Scholar]
- 32.Yeagle PL. Cholesterol and the cell-membrane. Biochim. Biophy. Acta. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 33.Brod J. Characterization and physiological-role of epidermal lipids. Int. J. Dermatol. 1991;30:84–90. doi: 10.1111/j.1365-4362.1991.tb04216.x. [DOI] [PubMed] [Google Scholar]
- 34.Middleton JD. Mechanism of action of surfactants on water binding properties of isolated stratum corneum. J. Soc. Cosmet. Chem. 1969;20:399–412. [Google Scholar]