Abstract
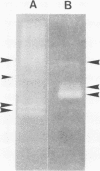
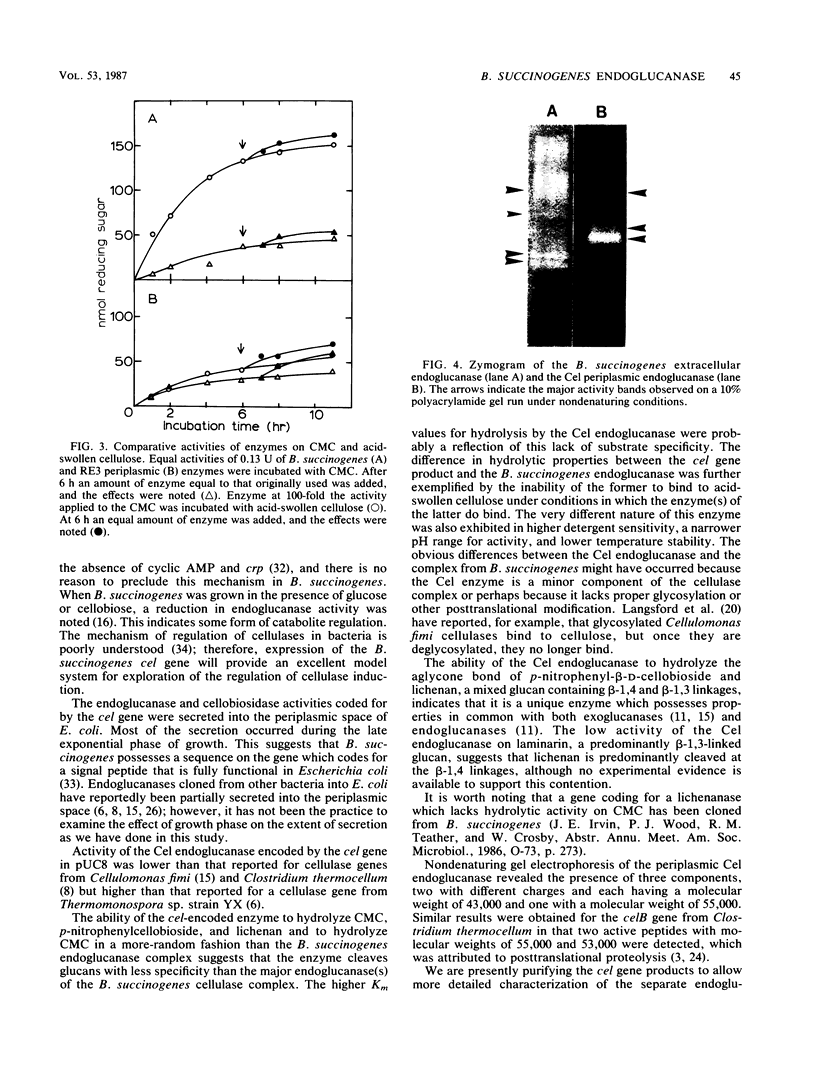
A cel gene from Bacteroides succinogenes inserted into the vector pUC8 coded for an enzyme which exhibited high hydrolytic activity on carboxymethylcellulose, p-nitrophenylcellobioside, and lichenan and low activity on laminarin and xylan. The enzyme was not synthesized by the Escherichia coli host when cells were cultured in complex medium containing added glucose. In the absence of added glucose, the endoglucanase and cellobiosidase activities synthesized were partitioned into the periplasmic space during growth, and practically all enzyme was located in the periplasm when the stationary phase of growth was reached. The enzyme exhibited 17- and sixfold higher Km values for the hydrolysis of carboxymethylcellulose and lichenan, respectively, than did the extracellular endoglucanase complex from B. succinogenes. The Cel endoglucanase had a pH optimum similar to that of the B. succinogenes enzyme except that the range was narrower, and the Cel endoglucanase was more readily inactivated on exposure to high temperature, detergents, and certain metals. Its activity was stimulated by calcium and magnesium. Nondenaturing polyacrylamide gel electrophoresis at different acrylamide concentrations revealed the presence of three endoglucanase components, two with molecular weights of 43,000 and one with a molecular weight of 55,000.
Full text
PDF
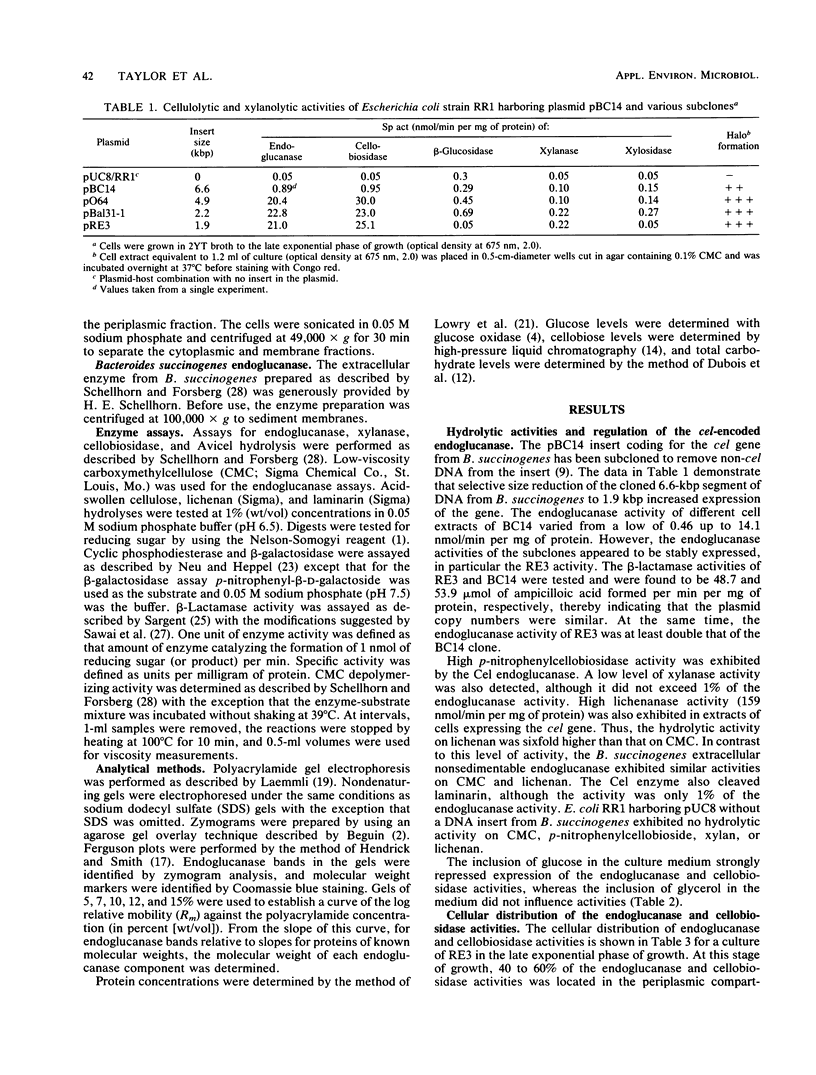
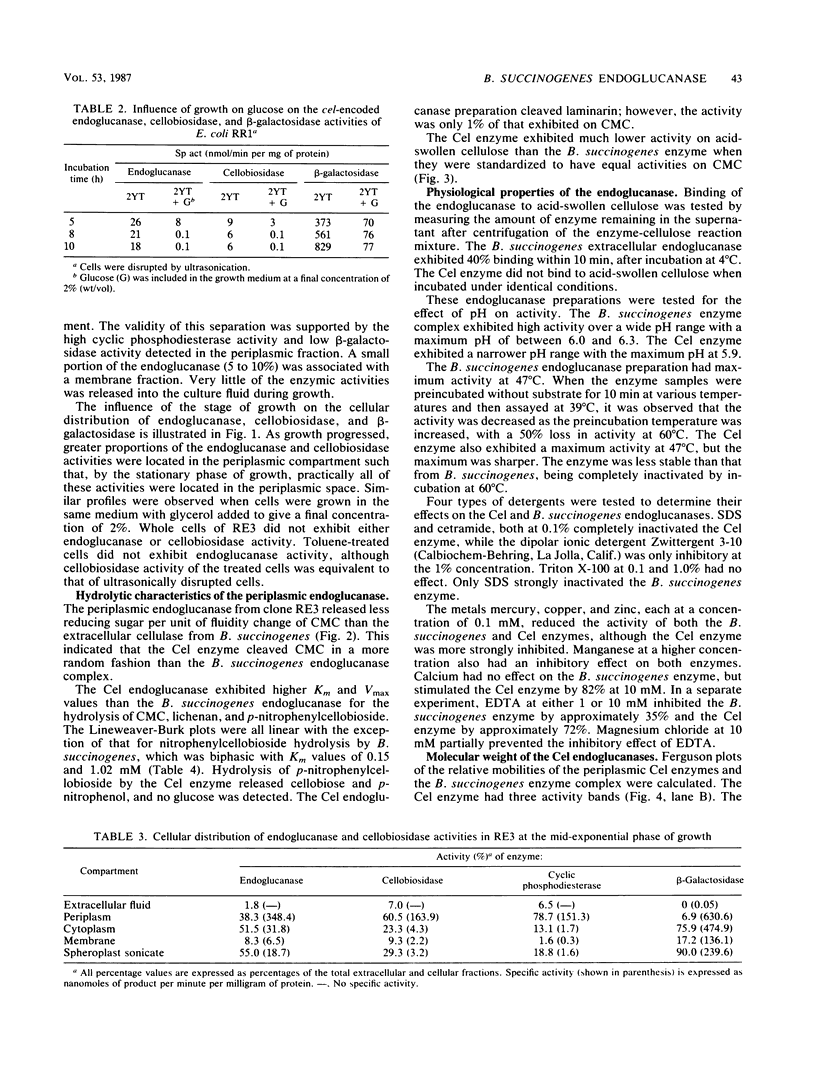
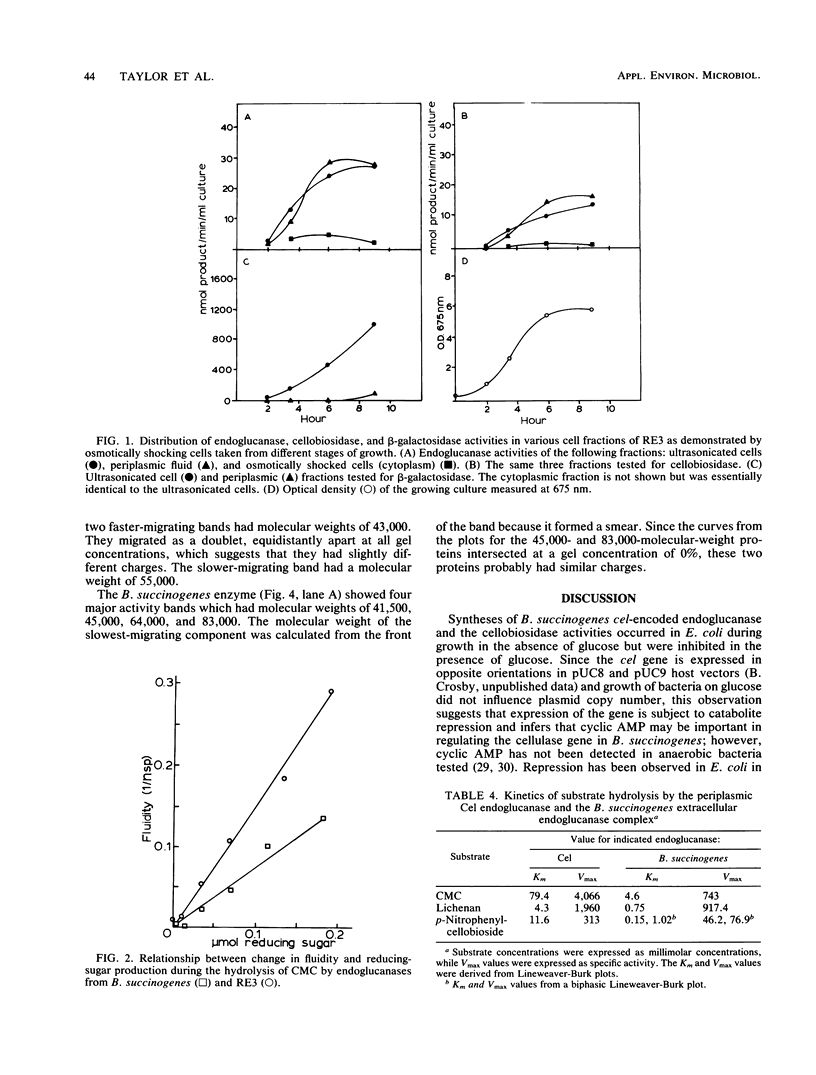

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beguin P., Cornet P., Millet J. Identification of the endoglucanase encoded by the celB gene of Clostridium thermocellum. Biochimie. 1983 Aug-Sep;65(8-9):495–500. doi: 10.1016/s0300-9084(83)80131-x. [DOI] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Cornelis P., Digneffe C., Willemot K. Cloning and expression of a Bacillus coagulans amylase gene in Escherichia coli. Mol Gen Genet. 1982;186(4):507–511. doi: 10.1007/BF00337957. [DOI] [PubMed] [Google Scholar]
- Deshpande V., Eriksson K. E., Pettersson B. Production , purification and partial characterization of 1,4-beta-glucosidase enzymes from Sporotrichum pulverulentum. Eur J Biochem. 1978 Sep 15;90(1):191–198. doi: 10.1111/j.1432-1033.1978.tb12590.x. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Crosby B., Thomas D. Y. Potential for manipulation of the rumen fermentation through the use of recombinant DNA techniques. J Anim Sci. 1986 Jul;63(1):310–325. doi: 10.2527/jas1986.631310x. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Langsford M. L., Kilburn D. G., Miller R. C., Jr, Warren R. A. Mode of action and substrate specificities of cellulases from cloned bacterial genes. J Biol Chem. 1984 Aug 25;259(16):10455–10459. [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Petre J., Longin R., Millet J. Purification and properties of an endo-beta-1,4-glucanase from Clostridium thermocellum. Biochimie. 1981 Jul;63(7):629–639. doi: 10.1016/s0300-9084(81)80061-2. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashihara N., Kudo T., Horikoshi K. Molecular cloning and expression of cellulase genes of alkalophilic Bacillus sp. strain N-4 in Escherichia coli. J Bacteriol. 1984 May;158(2):503–506. doi: 10.1128/jb.158.2.503-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Takahashi I., Yamagishi S. Iodometric assay method for beta-lactamase with various beta-lactam antibiotics as substrates. Antimicrob Agents Chemother. 1978 Jun;13(6):910–913. doi: 10.1128/aac.13.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Sacks L. E. Cyclic AMP is not detectable in Clostridium perfringens. Can J Microbiol. 1983 Sep;29(9):1228–1230. doi: 10.1139/m83-189. [DOI] [PubMed] [Google Scholar]
- Siegel L. S., Hylemon P. B., Phibbs P. V., Jr Cyclic adenosine 3',5'-monophosphate levels and activities of adenylate cyclase and cyclic adenosine 3',5'-monophosphate phosphodiesterase in Pseudomonas and Bacteroides. J Bacteriol. 1977 Jan;129(1):87–96. doi: 10.1128/jb.129.1.87-96.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Wood W. E., Neubauer D. G., Stutzenberger F. J. Cyclic AMP levels during induction and repression of cellulase biosynthesis in Thermomonospora curvata. J Bacteriol. 1984 Dec;160(3):1047–1054. doi: 10.1128/jb.160.3.1047-1054.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]