Abstract
Apoptosis occurs in response to different cellular stresses, including viral infection, inflammatory cytokines, growth factor deprivation, and UV light, but it is unclear whether these inducers share a common mechanism of induction. The interferon-induced, double-stranded RNA-activated protein kinase (PKR) has been implicated in processes that rely on apoptosis as control mechanisms in vivo, including antiviral activities, cell growth regulation, and tumorigenesis. Here we report that mouse embryo fibroblasts from mutant mice containing homozygous deletions in the PKR gene (Pkro/o mice) were resistant to apoptotic cell death in response to double-stranded RNA, tumor necrosis factor-α, or lipopolysaccharide. The mechanism underlying the suppression of apoptosis in the Pkro/o cells could be attributed to defects in the activation of DNA-binding activity for the transcription factor interferon regulatory factor-1 and in Fas mRNA induction. Thus, these results provide genetic evidence implicating a requirement for PKR in mediating different forms of stress-related apoptosis.
The process of programmed cell death or apoptosis is activated as a defensive mechanism for eliminating damaged or harmful cells within a multicellular organism. A highly conserved apoptotic protease cascade, comprised of members of the interleukin-1 converting enzyme-related family of proteases, represents the effector pathway for most, if not all, forms of apoptosis in metazoan cells (1). Apoptotic cell death can be induced by a great variety of signals, including cellular stress or DNA damage, but the signaling pathways linking these stimuli to the apoptotic protease cascade remain to be elucidated. Proposed mediators of the apoptotic responses to stress-related stimuli, such as tumor necrosis factor-α (TNF-α), lipopolysaccharide (LPS), and UV light, include ceramide production resulting from phospholipid hydrolysis and the activation of stress-activated protein kinase cascades, leading subsequently to the phosphorylation of transcription factors such as c-Jun or ATF2 (2–5).
The mechanisms and signaling mediators that regulate virus-induced apoptosis are less well understood, but it has long been established that a combination of interferon (IFN) and double-stranded RNA (dsRNA) is cytotoxic to cell cultures in vitro (6). dsRNA-activated protein kinase (PKR) is an IFN-inducible, dsRNA-activated serine/threonine kinase and one of the best studied mediators of cellular responses to virus infections. PKR can mediate inhibition of protein synthesis through phosphorylation of eukaryotic initiation factor-2α (eIF-2α), which, in the course of a viral infection, represents a defensive mechanism for restricting viral protein translation and, ultimately, viral replication. In addition, PKR can function as a signal transducer for mediating transcriptional activation in response to dsRNA via its ability to phosphorylate the inhibitor of nuclear factor-κB (IκB), resulting in the activation of nuclear factor-κB (NF-κB) (7, 8).
Mice with homozygous targeted deletion in the Pkr gene (Pkro/o mice), while apparently healthy in general, exhibited impaired antiviral responses after treatment with dsRNA or IFN-γ (9). Using mouse embryo fibroblasts (MEFs) derived from Pkro/o mice, it also has been shown that the absence of PKR results in deficient transcriptional responses to dsRNA and IFNs (10). Given the impaired antiviral responses in Pkro/o mice and the observations that overexpression of PKR is growth inhibitory in yeast (8, 11) and induces apoptotic death in HeLa cells (12), we investigated a role for PKR in mediating apoptosis using Pkro/o MEFs.
MATERIALS AND METHODS
Cell Culture and Apoptosis Assays.
MEFs from wild-type Pkr+/+ or Pkro/o mice (mixed C57/129sv backgrounds) were isolated as described previously and cultured in α-MEM (GIBCO) containing 10% fetal calf serum and antibiotics (9). For apoptosis assays, 2 × 105 cells in 1 ml of medium were plated and cultured overnight in 12-well plates, before treatment with the inducers poly(I)·poly(C) (pIC; Sigma), LPS (Sigma), TNF-α (Boehringer Mannheim), etoposide (Sigma), 5-fluorouracil (Sigma), or actinomycin D (Sigma) added directly to the medium. Cell viability after 24-h treatment was measured on trypsin-dispersed cells, using trypan blue dye exclusion as criterion. For detecting pycnotic nuclei, cells were grown as above on glass coverslips for 16 h, fixed with paraformaldehyde (1%), permeabilized with Nonidet P-40 (0.5%), stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma), and examined by fluorescent microscopy.
Electrophoretic Mobility Shift Assay.
MEFs (2.5 × 106) were cultured overnight before stimulation with the indicated inducers. The cells were harvested by scraping, washed once with PBS, and resuspended in extraction buffer (20 mM Tris·HCl, pH 8.0/150 mM NaCl/1.5 mM MgCl2/1% Triton X- 100/10% glycerol) for 20 min on ice. The samples were cleared by centrifugation (18,000 × g, 20 min, 4°C), and the supernatants, representing whole cell extracts, were collected. Electrophoretic mobility shift assays involved incubation of whole cell extract (10 μg protein) in binding buffer (8 mM Hepes, pH 7.0/10% glycerol/20 mM KCl/4 mM MgCl2/1 mM sodium pyrophosphate) containing 1.0 μg poly(dI)·poly(dC) and γ-32P-end-labeled probes (25 pg). The samples were analyzed by electrophoresis using 6% Tris·glycine polyacrylamide gels followed by autoradiography. Binding complexes corresponding to IFN regulatory factor (IRF)-1 and IRF-2 were inhibited by adding antibodies specific for these proteins (Santa Cruz Biotechnology) to the binding reactions. The IRF-1 antibody also was used to detect total IRF-1 protein in Western blot analysis.
Reverse Transcription–PCR.
Total RNA (1 μg) was reverse-transcribed using poly(dT) primer and Superscript II reverse transcriptase (GIBCO) in 25 μl of 50 mM Tris·HCl, pH 8.3/75 mM KCl/3 mM MgCl2/10 mM DTT/1 mM dNTP. PCR was performed on 2-μl aliquots from each cDNA reaction, using primer sets for detecting Fas (5′-CATCTCCGAGAGTTTAAAGCTGAGG, 5′-GTTTCCTGCAGTTTGTATTGCTGGTTGC) or glyceraldehyde-3-phosphate dehyrdogenase (GAPDH) (5′-CCATGGAGAAGGCTGGGG, 5′-CAAAGTTGTCATGGATGACC).
RESULTS
Pkro/o MEFs Are Resistant to Stress-Induced Apoptosis.
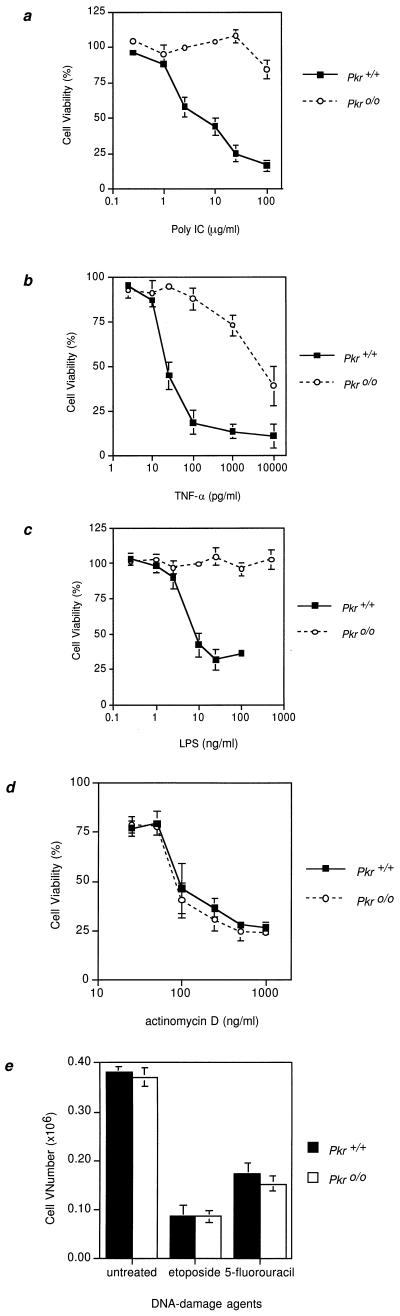
Treatment of MEFs from wild-type mice (Pkr+/+) with actinomycin and pIC resulted in cell death, which was characterized by morphological changes, such as pycnotic nuclei, a hallmark of apoptosis (Fig. 1 a and b). Pkr+/+ MEFs exhibited a dose-dependent apoptotic response to pIC with an LD50 of ≈2.5 μg/ml (Fig. 2a). Similar results were observed using mouse fibroblast (L929) or macrophage (RAW 264.7) cell lines (data not shown). In contrast, MEFs derived from Pkro/o mice were resistant to dsRNA-induced apoptosis even at 100 μg/ml of pIC (Fig. 2a) and did not show characteristic apoptotic features, indicating that the absence of PKR results in deficient induction of apoptosis (compare Figs. 1 b and e). To determine whether PKR could be implicated in other apoptotic pathways, we examined the effect of TNF-α on Pkr+/+ and Pkro/o MEFs. The sensitivity of Pkr+/+ MEFs to TNF-α (LD50 ≈20 pg/ml) was similar to that usually seen with L929 cells, which are used in standard biological assays for detecting TNF-α. In contrast, Pkro/o cells exhibited substantial resistance to TNF-α-induced cell death, as evidenced by an LD50 (≈5,000 pg/ml) more than 200-fold higher than that of Pkr+/+ cells (Fig. 2b). These differences also were reflected in the lower frequency of pycnotic nuclei observed in TNF-α-treated Pkro/o cells (compare Figs. 1 c and f). Because PKR has been previously implicated in mediating induction of genes by LPS (13–15) and because LPS also activates similar signaling pathways as TNF-α (e.g. stress-activated kinase activation and ceramide generation; refs. 16, 17), we investigated whether induction of apoptosis in response to LPS was impaired in Pkro/o fibroblasts. Whereas LPS induced a dose-dependent apoptotic response in Pkr+/+ MEFs (LD50, ≈5.0 ng/ml), Pkro/o MEFs exhibited nearly complete resistance to LPS, even at a concentration of 500 ng/ml (Fig. 2c). All of the above experiments were conducted in the presence of actinomycin D. As in most fibroblast cell types treated with TNF-α, the apoptotic effects described here are only fully realized in the presence of actinomycin D (18). However, the deficient apoptotic response of Pkro/o cells is not due to reduced sensitivity to actinomycin D. While the effective concentration of actinomycin D required to facilitate apoptosis was merely cytostatic (50 ng/ml), increasing doses were equally cytotoxic for either cell line (Fig. 2d). Despite the differences noted in stress-induced apoptosis, both Pkr+/+ and Pkro/o cells also exhibited equivalent sensitivity to the DNA-damaging agents, etoposide and 5-fluorouracil (Fig. 2e). Therefore, while PKR appears to be necessary for mediating stress-related apoptosis, its function is not required for DNA damage-related responses, and specific apoptotic mechanisms induced by DNA damage remain intact in Pkro/o MEFs.
Figure 1.
Decreased incidence of pycnotic nuclei in Pkro/o MEFs after exposure to apoptosis-inducing agents. (a–c) Pkr+/+ or (d–f) Pkro/o MEFs were treated with pIC (100 μg/ml) or TNF-α (1.0 ng/ml) as indicated, in the presence of actinomycin D (50 ng/ml) for 16 h. The cells then were stained with 4′,6-diamidino-2-phenylindole (DAPI) to distinguish normal from apoptotic nuclei. Treatment of cells with these inducers in the absence of actinomycin D did not induce any detectable apoptosis (data not shown).
Figure 2.
Dose responses of Pkr+/+ and Pkro/o MEFs to apoptosis-inducing agents. The viability of Pkr+/+ and Pkro/o MEFs was measured after exposure to the indicated concentrations of pIC (a), TNF-α (b), or LPS (c) in the presence of actinomycin D (50 ng/ml). Cell viability was determined by trypan blue dye exclusion after 24 h of treatment and is expressed as the percentage of viable cells relative to the control cultures treated with actinomycin D alone. Each data point represents the mean of three independent experiments with SD shown. (d) Sensitivity to increasing concentrations of actinomycin D is similar between Pkr+/+ and Pkro/o MEFs. (e) Pkr+/+ and Pkro/o MEFs show similar sensitivities to the DNA-damaging agents, etoposide (5 μM) and 5-fluorouracil (25 μM). Cell viability was measured 48 h after treatment with these agents.
Deficient IRF-1 DNA-Binding Activity in Pkro/o MEFs.
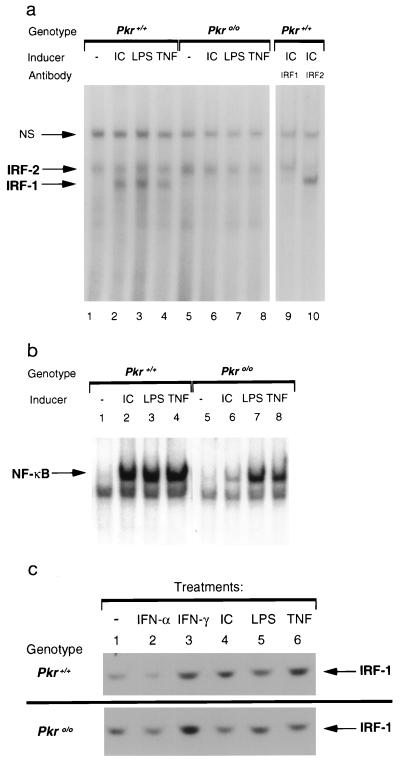
To investigate the mechanisms underlying PKR-mediated apoptosis, we examined the activation of IRF-1 in Pkro/o MEFs. IRF-1 can mediate transcriptional activity via specific cis-acting elements resident in the promoters of IFN and IFN-stimulated genes, although it is not essential for these processes (19–21). Homozygous deletion of Irf-1 results in permissiveness for transformation by c-Ha-ras in MEFs, and these transformed cells also exhibit resistance to apoptosis (22). To examine whether PKR may be implicated in IRF-1-mediated activities, electrophoretic mobility shift assays were performed with the multimerized hexamer probe, (AAGTGA)4, originally used to clone Irf-1 (named C1; ref. 23). Treatment of Pkr+/+ MEFs with pIC, TNF-α, or LPS induced IRF-1 binding activity, which was otherwise undetectable in untreated cells (Fig. 3a). In contrast, IRF-1 activation was impaired in Pkro/o cells treated with these same inducers. Comparable levels of constitutive IRF-2 binding activity was detectable in both Pkr+/+ and Pkro/o cells, and these levels were not modulated by any of the above inducers. These complexes were identified as IRF-1 and IRF-2 by using antibodies that specifically abolished the DNA-binding ability of each protein (Fig. 3a, lanes 9 and 10). Identical results were observed using an interferon-stimulated response element probe, which also binds IRF-1 and IRF-2 (data not shown). The lack of IRF-1 activation in Pkro/o cells by TNF-α or LPS does not reflect a global unresponsiveness to these inducers, because both were able to induce NF-κB activation in either cell type (Fig. 3b and ref. 9). These results are also consistent with recent studies indicating that NF-κB activation by TNF-α is not required for apoptosis and that a distinct signaling pathway mediates TNF-α-induced apoptosis (24). Finally, IRF-1 protein levels, as detected by Western blot analyses, are similar in Pkr+/+ and Pkro/o cells (Fig. 3c), suggesting that posttranslational mechanisms involving PKR likely regulate the activation of IRF-1.
Figure 3.
Detection of IRF-1 and NF-κB binding activities. Pkr+/+ or Pkro/o MEFs were stimulated with the indicated inducers for 4 h. Whole cell extracts were prepared and analyzed by electrophoretic mobility shift assay using (a) the C1 hexamer probe to detect IRF activation or (b) the PRDII probe to detect NF-κB activation. Arrows designate those complexes and a nonspecific complex (NS). (c) IRF-1 protein levels were detected by Western blot analyses.
Induction of Fas mRNA Is Deficient in Pkro/o MEFs.
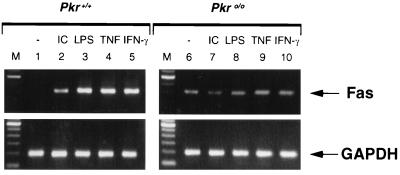
Given that IRF-1 activity is deficient in Pkro/o cells, we next examined candidate apoptosis genes whose expression may be affected as a consequence. The involvement of the Fas system in apoptosis for many cell types has been widely studied (25). Specifically, virus infection and pIC can stimulate transcription of Fas mRNA in fibroblasts (26). Treatment of Pkr+/+ MEFs with pIC, LPS, or TNF-α resulted in the induction of Fas mRNA (Fig. 4), in accordance with other studies. By contrast, no such induction was evident in Pkro/o MEFs, although a low constitutive level of Fas mRNA was detectable (Fig. 4).
Figure 4.
Fas mRNA induction is deficient in Pkro/o cells. Pkr+/+ or Pkro/o MEFs were stimulated with the indicated inducers for 6 h. Fas mRNA levels were detected by reverse transcription–PCR (35 cycles) and analysed using 2% agarose gel electrophoresis. Detection of glyceraldehyde-3-phosphate dehyrdogenase (GAPDH) mRNA by reverse transcription–PCR (30 cycles) was used as a control for general RNA integrity and equivalent loading.
DISCUSSION
This study identifies PKR as an essential mediator for several forms of stress-induced apoptosis. Specifically, our results implicate PKR in a signaling pathway that is responsive not only to dsRNA, but also to TNF-α and LPS. While the activation of PKR by dsRNA has been well studied (8), the mechanism of activation by these other stimuli is presently unclear. One possibility could involve the phosphorylation of PKR by an upstream kinase that is activated by one of the above stimuli [for example, the TNF-receptor-associated kinase, RIP (27), or a TNF-α/LPS-activated MAP kinase (4)]. Regardless of the activation mechanisms involved, our data show that PKR is required for regulating the DNA-binding ability of IRF-1 in response to stress-related stimuli. Previous studies have suggested that expression of IRF-1 protein in cells is insufficient to manifest any functional activity unless a phosphorylation signal is provided, potentially by PKR (28). While the mechanistic details of the interaction between PKR and IRF-1 remain to be elucidated, the deficient apoptosis in Irf-1o/o cells (22, 29) and the impaired IRF-1 activation in Pkro/o cells provides genetic evidence linking these two genes in a common apoptotic signaling pathway.
What are the possible contributions of the other known PKR substrates, eIF-2α and IκB, in mediating apoptosis? Phosphorylation of eIF-2α by PKR has been well studied as a mechanism leading to translation inhibition (8). A requirement for inhibition of new protein synthesis has been described in many studies regarding TNF-α-induced apoptosis (18). Most cells do not undergo significant apoptosis in response to TNF-α treatment alone unless they are simultaneously treated with an inhibitor of transcription or translation, commonly actinomycin D or cycloheximide. Consequently, it has long been hypothesized that in addition to transmitting cell death signals, TNF-α induces the synthesis of protective proteins that can counteract the apoptotic signals (see below). In this model, therefore, translation inhibition by itself is not responsible for inducing apoptosis, but it merely facilitates programmed cell death by preventing the expression of protective genes. It remains to be determined whether there are any differences in the levels of eIF-2α phosphorylation between Pkr+/+ and Pkro/o cells under the treatment conditions used here. However, the concentration of actinomycin D used in this study to facilitate apoptosis is 20-fold less than that which is typically used to inhibit transcription. Therefore, global translation inhibition via PKR-mediated eIF-2α phosphorylation may not be necessary as long as there is sufficiently reduced expression of protective genes (incurred by even low concentrations of actinomycin D) to allow for apoptotic mechanisms to proceed. Alternatively, the low actinomycin D doses may not have any transcription inhibitory effects, and they may be stimulating yet unidentified signaling pathways, such as DNA-damage responses, which then cooperate with PKR-mediated apoptotic signals. The necessity for some form of a costimulatory signal(s) also is represented in other examples of apoptosis involving primary fibroblasts. In particular, the apoptotic deficiencies described in p53o/o and Irf-1o/o MEFs were revealed by studying cells that initially were transfected with an oncogene, namely E1A or c-Ha-ras, respectively (29, 30). Oncogenic transformation reportedly acts to sensitize fibroblasts to apoptosis induced by DNA-damaging stimuli (30). While we did not detect any differences in the response of wild-type and Pkro/o cells to etoposide and 5-fluorouracil, it remains possible that the presence of a transforming oncogene may reveal different characteristics.
PKR also mediates activation of NF-κB via phosphorylation of IκB. Recently, several studies have described a novel role for NF-κB in protection against apoptosis induced by TNF-α and chemotherapeutic agents. Specifically, cells lacking functional NF-κB (as the result of overexpression of a mutant IκBα super-repressor or homozygous deletion of RelA) were observed to be more sensitive to TNF-α-induced cell death (31–34). Thus, these data support and extend the hypothesis discussed earlier whereby TNF-α induces the expression of putative protective genes through NF-κB-dependent mechanisms. Because our results indicate that NF-κB activation by TNF-α or LPS appears to be unaffected by the absence of PKR, the diminished apoptotic responses to these inducers in Pkro/o cells likely results from a deficiency in PKR-dependent apoptotic death signals. This is further supported by our results using dsRNA as an apoptosis inducer. As NF-κB activation by dsRNA is deficient in Pkro/o cells (ref. 9 and Fig. 3b), these cells might have been predicted to be more sensitive to apoptosis simply because NF-κB-dependent protective genes cannot be induced. However, the finding that Pkro/o cells are actually resistant to dsRNA-induced cell death is consistent with PKR having an effector function in mediating apoptosis.
Significant advances have been made in the identification of many cellular proteins that associate with the TNF receptor and their respective roles in mediating different arms of downstream TNF signaling (1, 24, 31). Specifically, FADD was identified as having an important role in mediating TNF-α-induced apoptosis. Because Pkro/o cells undergo apoptosis in response to high doses of TNF-α, it is clear that some TNF-α-induced apoptotic pathways are functional in the absence of PKR. Presently, it is unknown whether PKR has any impact on the activities of TNF-receptor-associated components or if PKR may have an auxiliary role in modulating further downstream aspects of TNF signaling. Alternatively, PKR-mediated apoptotic signaling may represent an independent pathway that is primarily responsive to dsRNA or LPS, but which happens to be coactivated by the TNF-receptor complex and thereby augments the basic TNF-α-induced apoptotic signals. Further studies are required to address these issues. Additional support for a substantive role of PKR in apoptosis was provided by recent observations that stable U937 cell transfectants, containing an antisense PKR construct, were also less susceptible to TNF-α-induced cell death (35).
Finally, we note that Pkro/o mice appear to be generally healthy and do not exhibit any of the phenotypes (e.g., lymphadenopathy and splenomegaly) associated with mutant mice (derived from the lpr and gld mice strains), which bear homozygous mutations of the Fas and Fas ligand genes (25). Consequently, it is possible that while regulation of the Fas system in the lymphoid compartment does not require PKR, specific stress-induced responses in fibroblasts involving Fas are PKR-dependent.
Acknowledgments
We thank Maryam Zamanian-Daryoush for preparing the MEFs, and Aseem Kumar and Allan Lau for helpful discussions. This work was supported by a grant to B.R.G.W. from the National Institutes of Health (AI34039-02).
ABBREVIATIONS
- IFN
interferon
- dsRNA
double-stranded RNA
- PKR
dsRNA-activated protein kinase
- MEF
mouse embryo fibroblast
- IRF
IFN regulatory factor
- TNF-α
tumor necrosis factor-α
- pIC
poly(I)·poly(C)
- LPS
lipopolysaccharide
- eIF-2α
eukaryotic initiation factor-2α
- NF-κB
nuclear factor-κB
- IκB
inhibitor of NF-κB
References
- 1.Fraser A, Evan G. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 2.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Campbell D, Derijard B, Davis R J. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 4.Ulevitch R J, Tobias P S. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 5.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 6.Lengyel P. J Interferon Res. 1987;7:511–519. doi: 10.1089/jir.1987.7.511. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Haque J, Lacoste J, Hiscott J, Williams B R G. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams B R G. Semin Virol. 1995;6:191–202. [Google Scholar]
- 9.Yang Y L, Reis L F L, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R G, Aguet M, Weissmann C. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Yang Y-L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R G. EMBO J. 1996;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong K L, Feng L, Schappert K, Meurs E, Donahue T F, Friesen J D, Hovanessian A G, Williams B R G. EMBO J. 1992;11:1553–1562. doi: 10.1002/j.1460-2075.1992.tb05200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S B, Esteban M. Virology. 1994;199:491–496. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- 13.Der S D, Lau A S. Proc Natl Acad Sci USA. 1995;92:8841–8845. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gusella G L, Musso T, Rottschafer S E, Pulkki K, Varesio L. J Immunol. 1995;154:345–354. [PubMed] [Google Scholar]
- 15.Koromilas A E, Cantin C, Craig A W B, Jagus R, Hiscott J, Sonenberg N. J Biol Chem. 1995;270:25426–25434. doi: 10.1074/jbc.270.43.25426. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Lee J D, Bibbs L, Ulevitch R J. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 17.Hambleton J, Weinstein S L, Lem L, DeFranco A L. Proc Natl Acad Sci USA. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyaert R, Fiers W. FEBS Lett. 1994;340:9–16. doi: 10.1016/0014-5793(94)80163-0. [DOI] [PubMed] [Google Scholar]
- 19.Kimura T, Nakayama K, Penninger J, Kitagawa M, Harada H, Matsuyama T, Tanaka N, Kamijo R, Vilcek J, Mak T W, Taniguchi T. Science. 1994;264:1921–1924. doi: 10.1126/science.8009222. [DOI] [PubMed] [Google Scholar]
- 20.Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kundig T M, Amakawa R, Kishihara K, Wakeham A, Potter J, Furlonger C L, Narendran A, Suzuki H, Ohashi P S, Paige C J, Taniguchi T, Mak T W. Cell. 1993;75:83–97. [PubMed] [Google Scholar]
- 21.Reis L, Ruffner H, Stark G, Aguet M, Weissmann C. EMBO J. 1994;13:4798–4806. doi: 10.1002/j.1460-2075.1994.tb06805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier M S, Aizawa S, Mak T W, Taniguchi T. Cell. 1994;77:829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 24.Hsu H, Shu H-B, Pan M-G, Goeddel D V. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 25.Nagata S, Suda T. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 26.Wada N, Matsumura M, Ohba Y, Kobayashi N, Takizawa T, Nakanishi Y. J Biol Chem. 1995;270:18007–18012. doi: 10.1074/jbc.270.30.18007. [DOI] [PubMed] [Google Scholar]
- 27.Stanger B Z, Leder P, Lee T-H, Kim E, Seed B. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe N, Sakakibara J, Hovanessian A G, Taniguchi T, Fujita T. Nucleic Acids Res. 1992;19:4421–4428. doi: 10.1093/nar/19.16.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura T, Ishihara M, Lamphier M S, Tanaka N, Oishi I, Aizawa S, Matsuyama T, Mak T W, Taki S, Taniguchi T. Nature (London) 1995;376:596–599. doi: 10.1038/376596a0. [DOI] [PubMed] [Google Scholar]
- 30.Lowe S W, Ruley H E, Jacks T, Housman D E. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z-G, Hsu H, Goeddel D V, Karin M. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 32.Wang C-Y, Mayo M W, Baldwin A S. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 33.Beg A A, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 34.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 35.Yeung M C, Liu J, Lau A S. Proc Natl Acad Sci USA. 1996;93:12451–12455. doi: 10.1073/pnas.93.22.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]