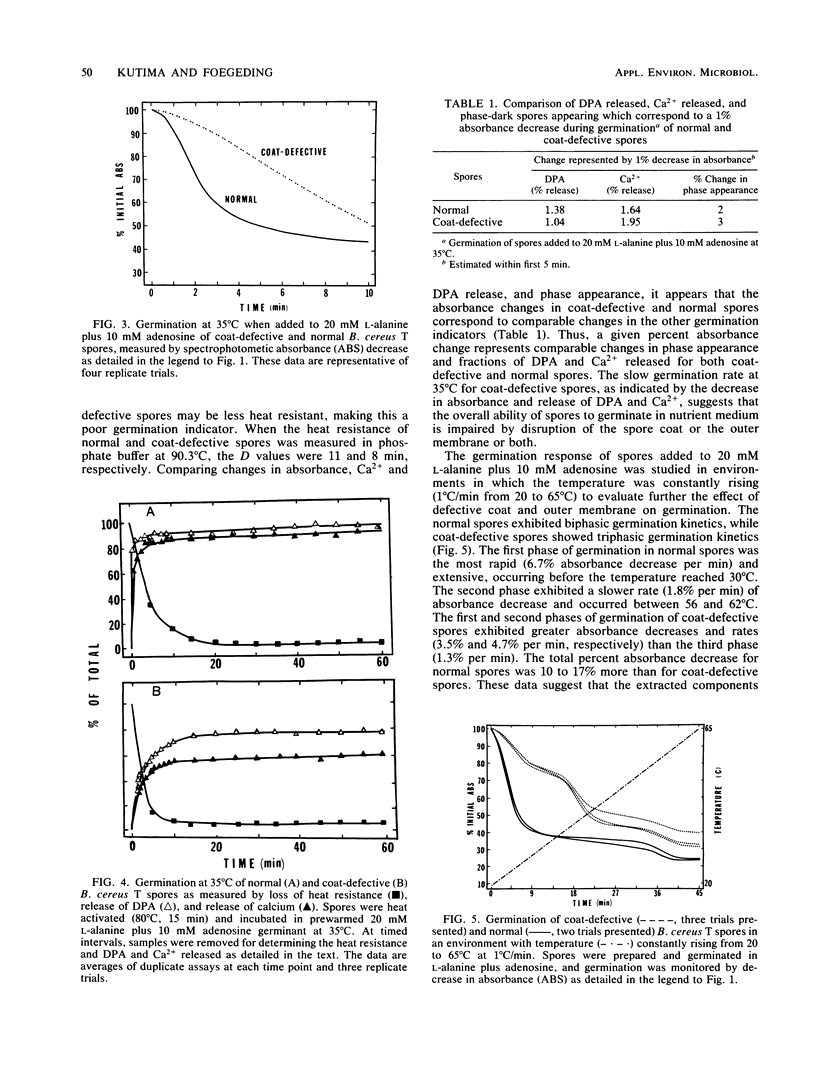
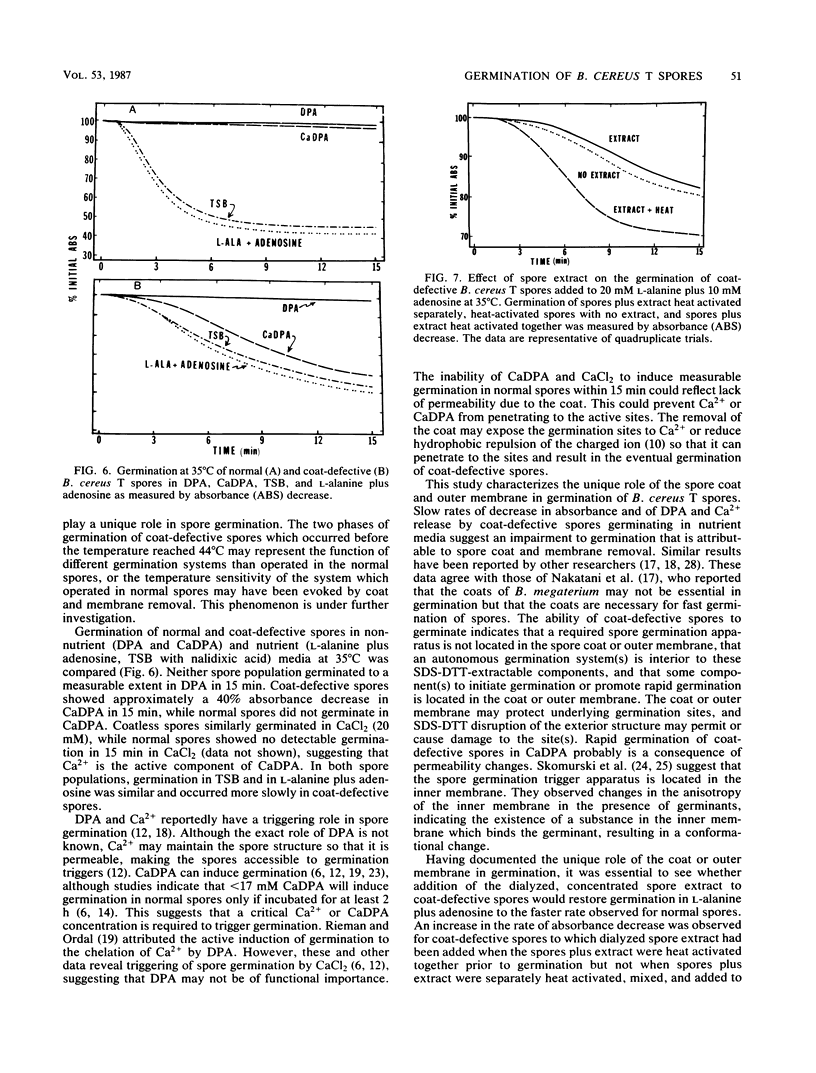
Abstract
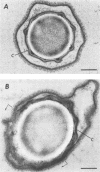
Bacillus cereus T spores were prepared on fortified nutrient agar, and the spore coat and outer membrane were extracted by 0.5% sodium dodecyl sulfate-100 mM dithiothreitol in 0.1 M sodium chloride (SDS-DTT) at pH 10.5 (coat-defective spores). Coat-defective spores in L-alanine plus adenosine germinated slowly and to a lesser extent than spores not treated with SDS-DTT, as determined by decrease in absorbance and release of dipicolinic acid and Ca2+. Spores germinated in calcium dipicolinate only after treatment with SDS-DTT. Biphasic and triphasic germination kinetics were observed with normal and coat-defective spores, respectively, in an environment with temperature increasing from 20 to 65 degrees C at a rate of 1 degree C/min. Therefore, the physical and biochemical processes involved in germination are modified by coat removal. The data suggest that a portion of the germination apparatus located interior to the coat may be protected by the coat and outer membrane or that the coat and outer membrane otherwise enhance germination in L-alanine plus adenosine. When coat-defective spores were heat activated with the dialyzed (12,000-Mr cutoff) components extracted from the spores, germination of the SDS-DTT-treated spores was enhanced; thus, one or more components located in the spore coat or outer membrane with a molecular weight greater than 12,000 were essential for fast germination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando Y. Spore lytic enzyme released from Clostridium perfringens spores during germination. J Bacteriol. 1979 Oct;140(1):59–64. doi: 10.1128/jb.140.1.59-64.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968 Apr 14;33(1):199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Reconstitution of bacterial spore coat layers in vitro. J Bacteriol. 1971 Oct;108(1):571–578. doi: 10.1128/jb.108.1.571-578.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman T. C., Pankratz H. S., Gerhardt P. Ultrastructure of the exosporium and underlying inclusions in spores of Bacillus megaterium strains. J Bacteriol. 1972 Mar;109(3):1198–1209. doi: 10.1128/jb.109.3.1198-1209.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassier M., Ryter A. Sur un mutant de Clostridium perfringens donnant des spores sans tuniques à germination lysozyme-dépendante. Ann Inst Pasteur (Paris) 1971 Dec;121(6):717–732. [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W., Dring G. J. Heat resistance of bacterial endospores and concept of an expanded osmoregulatory cortex. Nature. 1975 Dec 4;258(5534):402–405. doi: 10.1038/258402a0. [DOI] [PubMed] [Google Scholar]
- Kamat A. S., Lewis N. F., Pradhan D. S. Mechanism of Ca2+ and dipicolinic acid requirement for L-alanine induced germination of Bacillus cereus BIS-59 spores. Microbios. 1985;44(177):33–44. [PubMed] [Google Scholar]
- Koshikawa T., Beaman T. C., Pankratz H. S., Nakashio S., Corner T. R., Gerhardt P. Resistance, germination, and permeability correlates of Bacillus megaterium spores successively divested of integument layers. J Bacteriol. 1984 Aug;159(2):624–632. doi: 10.1128/jb.159.2.624-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Ordal Z. J. REVERSIBLE ACTIVATION FOR GERMINATION AND SUBSEQUENT CHANGES IN BACTERIAL SPORES. J Bacteriol. 1963 Jan;85(1):207–217. doi: 10.1128/jb.85.1.207-217.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y., Imagawa M., Takubo Y., Nishikawa J., Nishihara T., Kondo M. Germination of the decoated spores of Bacillus megaterium. Microbiol Immunol. 1985;29(12):1139–1149. doi: 10.1111/j.1348-0421.1985.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Nakatani Y., Tani K., Imagawa M., Nishihara T., Kondo M. Germinability of coat-lacking spores of Bacillus megaterium. Biochem Biophys Res Commun. 1985 Apr 30;128(2):728–732. doi: 10.1016/0006-291x(85)90107-x. [DOI] [PubMed] [Google Scholar]
- RIEMANN H., ORDAL Z. J. Germination of bacterial endospores with calcium and dipicolinic acid. Science. 1961 May 26;133(3465):1703–1704. doi: 10.1126/science.133.3465.1703. [DOI] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. Influence of exchangeable ions on germinability of bacterial spores. J Bacteriol. 1966 Apr;91(4):1582–1588. doi: 10.1128/jb.91.4.1582-1588.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I. R., Ellar D. J. Study of calcium dipicolinate release during bacterial spore germination by using a new, sensitive assay for dipicolinate. J Bacteriol. 1978 Jul;135(1):133–137. doi: 10.1128/jb.135.1.133-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelma G. N., Jr, Aronson A. I., Fitz-James P. C. A Bacillus cereus mutant defective in spore coat deposition. J Gen Microbiol. 1980 Jan;116(1):173–185. doi: 10.1099/00221287-116-1-173. [DOI] [PubMed] [Google Scholar]
- Stelma G. N., Jr, Aronson A. I., Fitz-James P. Properties of Bacillus cereus temperature-sensitive mutants altered in spore coat formation. J Bacteriol. 1978 Jun;134(3):1157–1170. doi: 10.1128/jb.134.3.1157-1170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973 Nov;116(2):797–802. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D. Liquid chromatographic determination of dipicolinic Acid from bacterial spores. Appl Environ Microbiol. 1979 Dec;38(6):1029–1033. doi: 10.1128/aem.38.6.1029-1033.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D. Molecular structure of the bacterial spore. Adv Microb Physiol. 1978;17:1–45. doi: 10.1016/s0065-2911(08)60056-9. [DOI] [PubMed] [Google Scholar]