Abstract
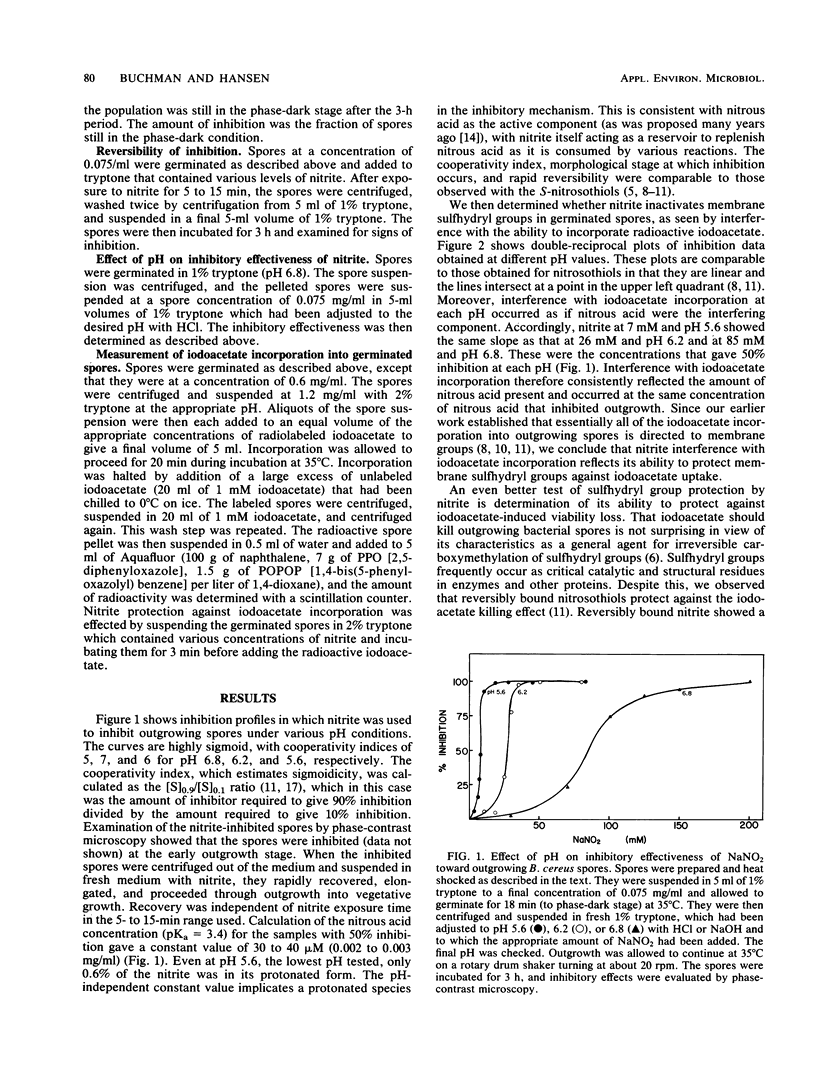
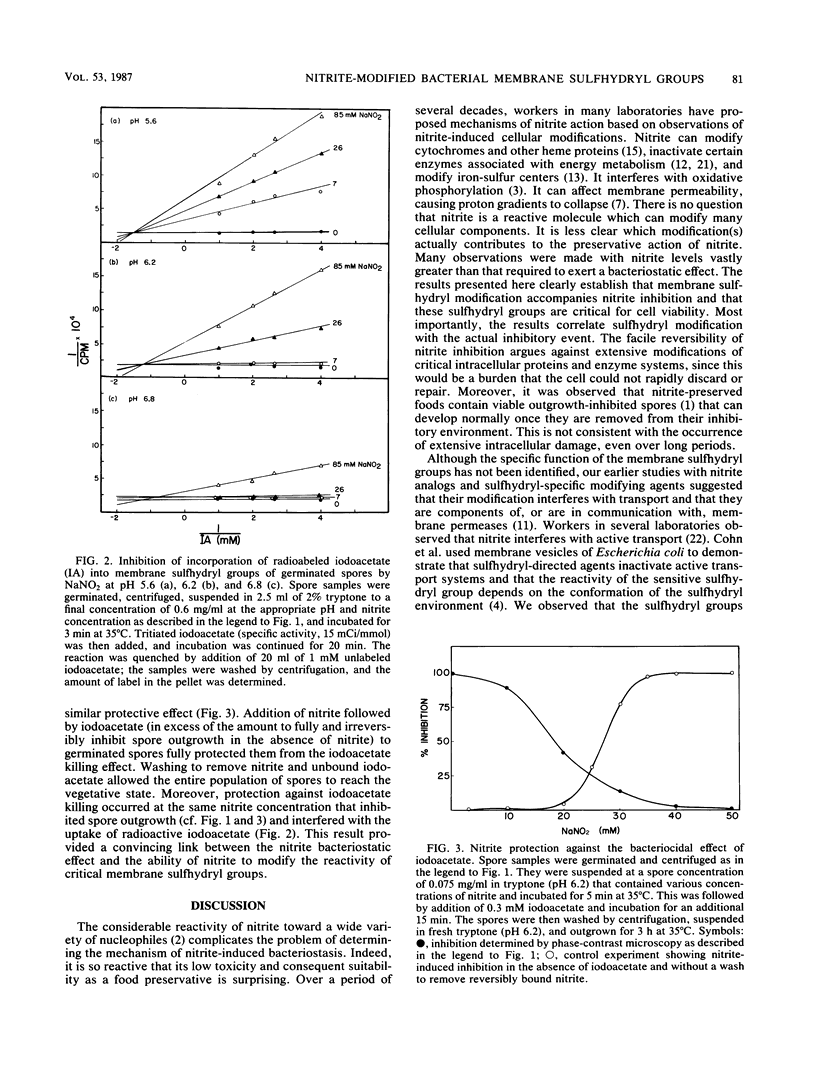
The mechanism by which nitrite inhibits outgrowing spores of Bacillus cereus T was examined by using techniques developed earlier for nitrite analogs. The morphological stage of inhibition, cooperativity effects, effect of pH on inhibition, kinetics of protection against iodoacetate incorporation into membrane sulfhydryl groups, and protection against the bacteriocidal effect of carboxymethylation by iodoacetate indicate that nitrite acts as a membrane-directed sulfhydryl agent. The mechanism by which nitrite modifies the chemical reactivity of the sulfhydryl group could be either direct covalent modification or inactivation through communication with another modified membrane component. Profiles of pH effects suggest that the active agent is the protonated form of nitrite. The nitrite concentrations which modify membrane sulfhydryl activity coincide with those which have a bacteriostatic effect. These results are consistent with membrane sulfhydryl modification as a component of the mechanism of nitrite-induced bacteriostasis in this aerobic sporeformer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn D. E., Kaczorowski G. J., Kaback H. R. Effect of the proton electrochemical gradient on maleimide inactivation of active transport in Escherichia coli membrane vesicles. Biochemistry. 1981 May 26;20(11):3308–3313. doi: 10.1021/bi00514a050. [DOI] [PubMed] [Google Scholar]
- Hansen J. N., Levin R. A. Effect of some inhibitors derived from nitrite on marcomolecular synthesis in Bacillus cereus. Appl Microbiol. 1975 Nov;30(5):862–869. doi: 10.1128/am.30.5.862-869.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer E. M., van der Zwaan J. W., Wever R., Stouthamer A. H. Anaerobic respiration and energy conservation in Paracoccus denitrificans. Functioning of iron-sulfur centers and the uncoupling effect of nitrite. Eur J Biochem. 1979 May 2;96(1):69–76. doi: 10.1111/j.1432-1033.1979.tb13014.x. [DOI] [PubMed] [Google Scholar]
- Morris S. L., Hansen J. N. Inhibition of Bacillus cereus spore outgrowth by covalent modification of a sulfhydryl group by nitrosothiol and iodoacetate. J Bacteriol. 1981 Nov;148(2):465–471. doi: 10.1128/jb.148.2.465-471.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. L., Walsh R. C., Hansen J. N. Identification and characterization of some bacterial membrane sulfhydryl groups which are targets of bacteriostatic and antibiotic action. J Biol Chem. 1984 Nov 10;259(21):13590–13594. [PubMed] [Google Scholar]
- O'Leary V., Solberg M. Effect of sodium nitrite inhibition on intracellular thiol groups and on the activity of certain glycolytic enzymes in Clostridium perfringens. Appl Environ Microbiol. 1976 Feb;31(2):208–212. doi: 10.1128/aem.31.2.208-212.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D., Lancaster J. R., Jr, Cornforth D. P. Nitrite inhibition of Clostridium botulinum: electron spin resonance detection of iron-nitric oxide complexes. Science. 1983 Aug 19;221(4612):769–770. doi: 10.1126/science.6308761. [DOI] [PubMed] [Google Scholar]
- VARY J. C., HALVORSON H. O. KINETICS OF GERMINATION OF BACILLUS SPORES. J Bacteriol. 1965 May;89:1340–1347. doi: 10.1128/jb.89.5.1340-1347.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L. F., Wood J. M. A note on the effect of nitrite inhibition on the metabolism of Clostridium botulinum. J Appl Bacteriol. 1982 Feb;52(1):109–110. doi: 10.1111/j.1365-2672.1982.tb04380.x. [DOI] [PubMed] [Google Scholar]
- Yarbrough J. M., Rake J. B., Eagon R. G. Bacterial inhibitory effects of nitrite: inhibition of active transport, but not of group translocation, and of intracellular enzymes. Appl Environ Microbiol. 1980 Apr;39(4):831–834. doi: 10.1128/aem.39.4.831-834.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



