Abstract
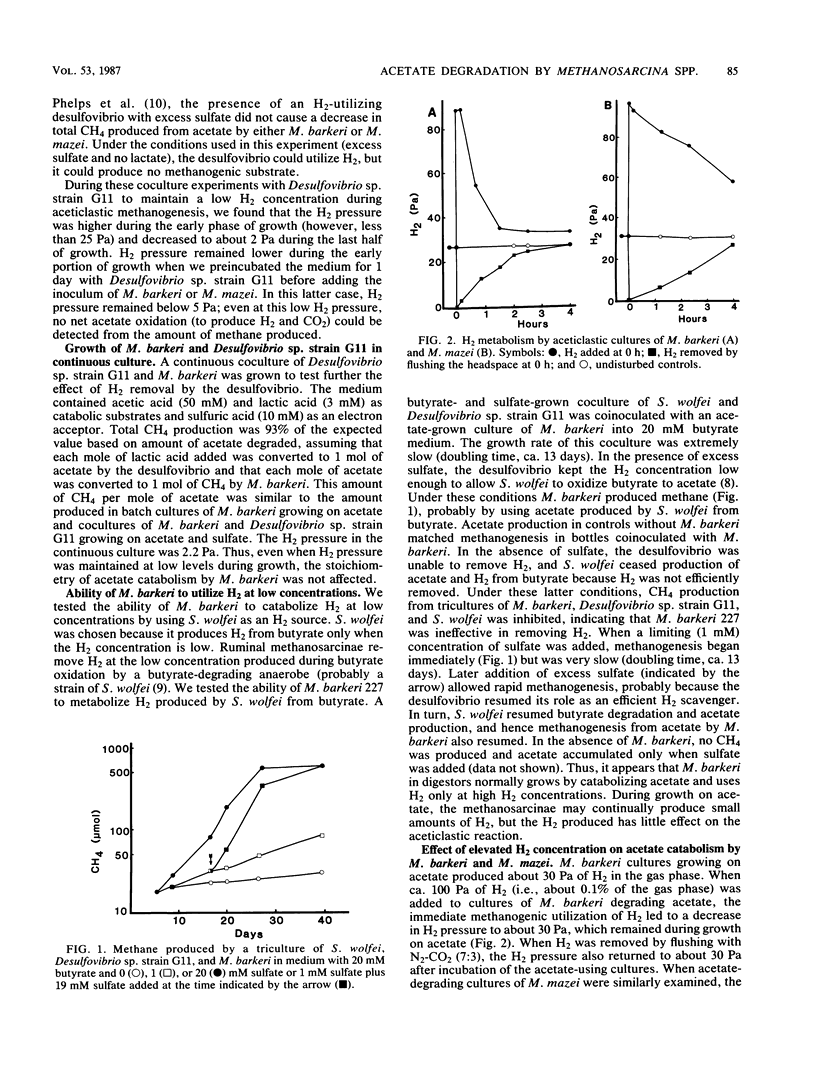
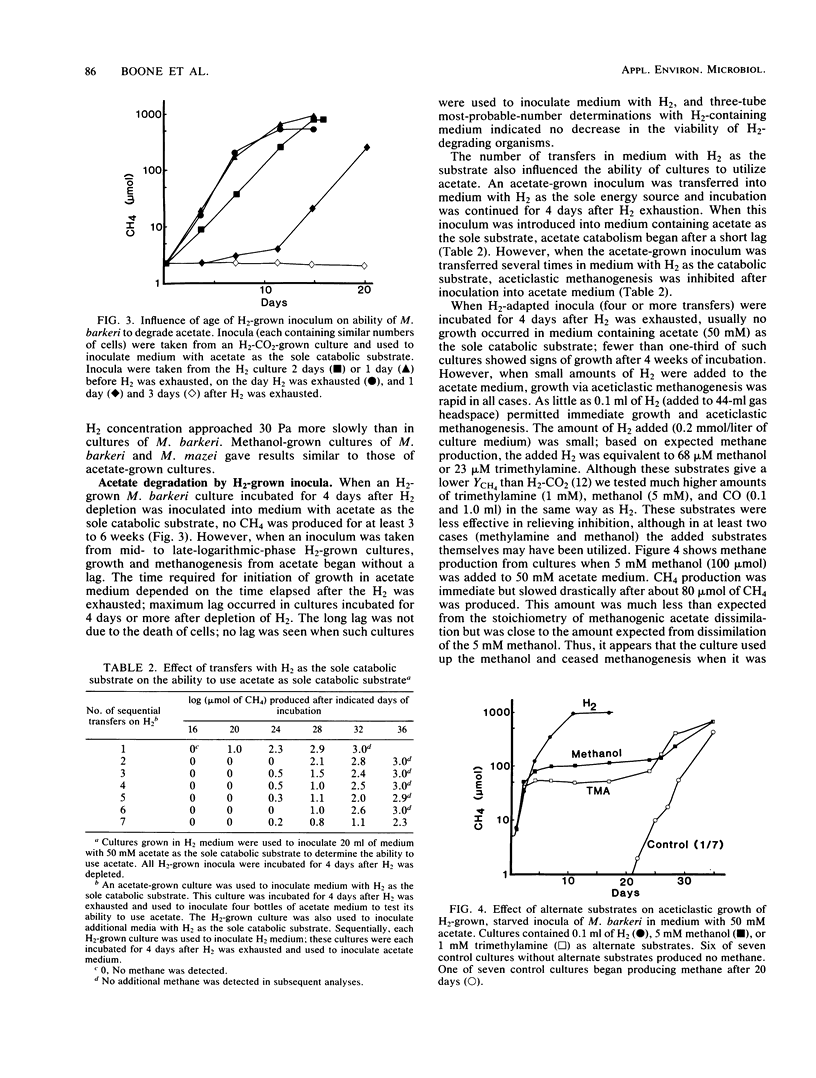
Methanosarcina barkeri 227 and Methanosarcina mazei S-6 grew with acetate as the substrate; we found little effect of H2 on the rate of aceticlastic growth in the presence of various H2 pressures between 2 and 810 Pa. We used physical (H2 addition or flushing the headspace to remove H2) and biological (H2-producing or -utilizing bacteria in cocultures) methods for controlling H2 pressure in Methanosarcina cultures growing on acetate. Added H2 (ca. 100 Pa) was removed rapidly (a few hours) by M. barkeri and slowly (within a day) by M. mazei. When the H2 produced by the aceticlastic methanogens was removed by coculturing with an H2-using Desulfovibrio sp., the H2 pressure was about 2.2 Pa. Under these conditions the stoichiometry of aceticlastic methanogenesis did not change. H2-grown inocula of M. barkeri grew with acetate as the sole catabolic substrate if the inoculum culture was transferred during logarithmic growth to acetate-containing medium or if the transfer was accomplished within 1 or 2 days after exhaustion of H2. H2-grown cultures incubated for 4 or more days after exhaustion of H2 were able to grow with H2 but not with acetate as the sole catabolic substrate. Addition of small quantities of H2 to acetate-containing medium permitted these cultures to initiate growth on acetate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R. Terminal reactions in the anaerobic digestion of animal waste. Appl Environ Microbiol. 1982 Jan;43(1):57–64. doi: 10.1128/aem.43.1.57-64.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson T. J., Mah R. A. Isolation and characterization of an h(2)-oxidizing thermophilic methanogen. Appl Environ Microbiol. 1983 Jan;45(1):265–274. doi: 10.1128/aem.45.1.265-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Ferry J. G. Production and Consumption of H(2) during Growth of Methanosarcina spp. on Acetate. Appl Environ Microbiol. 1985 Jan;49(1):247–249. doi: 10.1128/aem.49.1.247-249.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P. Anaerobic Degradation of Lactate by Syntrophic Associations of Methanosarcina barkeri and Desulfovibrio Species and Effect of H(2) on Acetate Degradation. Appl Environ Microbiol. 1981 Feb;41(2):346–354. doi: 10.1128/aem.41.2.346-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Mackie R. I., Bryant M. P. Syntrophic association of a butyrate-degrading bacterium and methanosarcina enriched from bovine rumen fluid. Appl Environ Microbiol. 1981 Mar;41(3):826–828. doi: 10.1128/aem.41.3.826-828.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps T. J., Conrad R., Zeikus J. G. Sulfate-Dependent Interspecies H(2) Transfer between Methanosarcina barkeri and Desulfovibrio vulgaris during Coculture Metabolism of Acetate or Methanol. Appl Environ Microbiol. 1985 Sep;50(3):589–594. doi: 10.1128/aem.50.3.589-594.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Growth and methanogenesis by Methanosarcina strain 227 on acetate and methanol. Appl Environ Microbiol. 1978 Dec;36(6):870–879. doi: 10.1128/aem.36.6.870-879.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


