Abstract
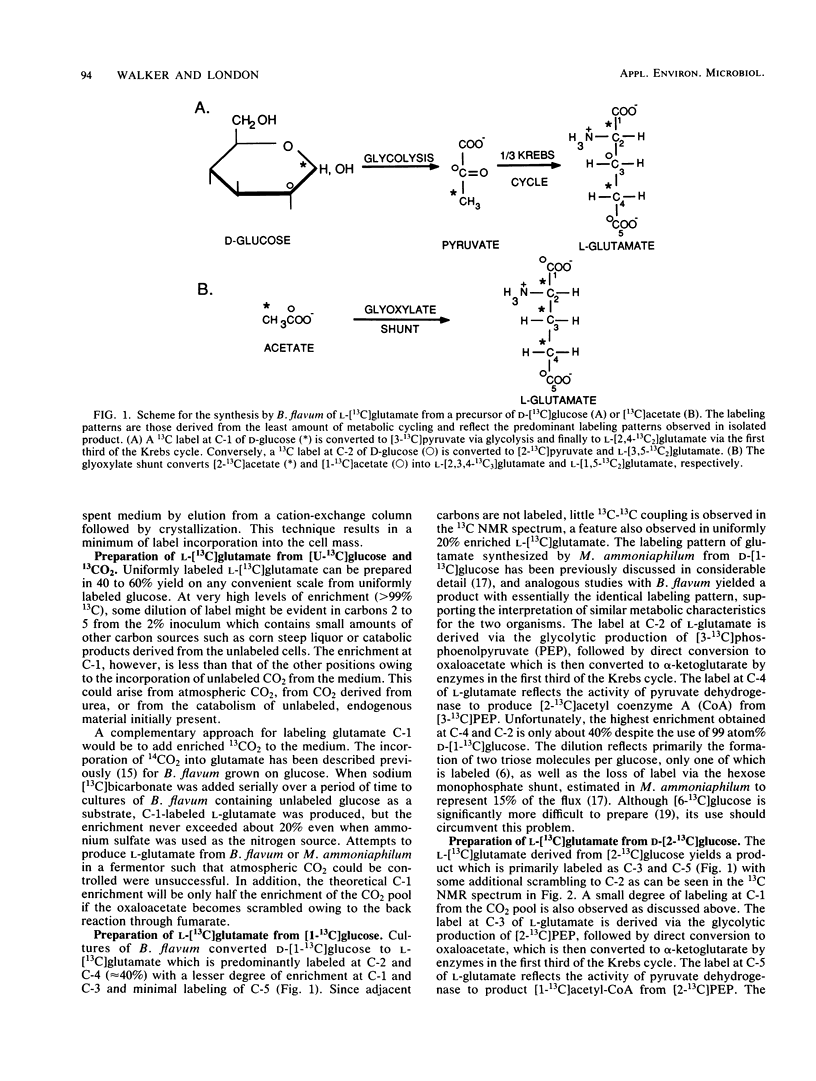
The biosynthesis of isotopically labeled L-glutamic acid by the microorganism Brevibacterium flavum was studied with a variety of carbon-13-enriched precursors. The purpose of this study was twofold: to develop techniques for the efficient preparation of labeled L-glutamate with a variety of useful labeling patterns which can be used for other metabolic studies, and to better understand the metabolic events leading to label scrambling in these strains. B. flavum, which is used commercially for the production of monosodium glutamate, has the capability of utilizing glucose or acetate as a sole carbon source, an important criterion from the standpoint of developing labeling strategies. Unfortunately, singly labeled glucose precursors lead to excessive isotopic dilution which reduces their usefulness. Studies with [3-13C]pyruvate indicate that this problem can in principle be overcome by using labeled three-carbon precursors; however, conditions could not be found which would lead to an acceptable yield of isotopically labeled L-glutamate. In contrast, [1-13C]- or [2-13C]acetate provides relatively inexpensive, readily available precursors for the production of selectively labeled, highly enriched L-glutamate. The preparation of L-[15N]glutamate from [15N]ammonium sulfate was carried out and is a very effective labeling strategy. Analysis of the isotopic distribution in labeled glutamate provides details about the metabolic pathways in these interesting organisms.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bojan O., Bologa M., Niac G., Palibroda N., Vargha E., Bârzu O. Simple enzymatic procedure for preparation of 15N-labeled L-glutamic acid. Anal Biochem. 1980 Jan 1;101(1):23–25. doi: 10.1016/0003-2697(80)90034-2. [DOI] [PubMed] [Google Scholar]
- LeMaster D. M., Cronan J. E., Jr Biosynthetic production of 13C-labeled amino acids with site-specific enrichment. J Biol Chem. 1982 Feb 10;257(3):1224–1230. [PubMed] [Google Scholar]
- London R. E., Walker T. E. Biosynthesis of trehalose by Brevibacterium flavum: use of long range 13C-13C coupling data to characterize triose phosphate isomerase activity. Biosci Rep. 1985 Jun;5(6):509–515. doi: 10.1007/BF01116950. [DOI] [PubMed] [Google Scholar]
- SHIIO I., OTSUKA S. I., KATSUYA N. Cellular permeability and extracellular formation of glutamic acid in Brevibacterium flavum. J Biochem. 1963 May;53:333–340. doi: 10.1093/oxfordjournals.jbchem.a127706. [DOI] [PubMed] [Google Scholar]
- SHIIO I., OTSUKA S. I., TAKAHASHI M. Effect of biotin on the bacterial formation of glutamic acid. I. Glutamate formation and cellular premeability of amino acids. J Biochem. 1962 Jan;51:56–62. doi: 10.1093/oxfordjournals.jbchem.a127500. [DOI] [PubMed] [Google Scholar]
- Walker T. E., Han C. H., Kollman V. H., London R. E., Matwiyoff N. A. 13C nuclear magnetic resonance studies of the biosynthesis by Microbacterium ammoniaphilum of L-glutamate selectively enriched with carbon-13. J Biol Chem. 1982 Feb 10;257(3):1189–1195. [PubMed] [Google Scholar]
- Walsh K., Koshland D. E., Jr Determination of flux through the branch point of two metabolic cycles. The tricarboxylic acid cycle and the glyoxylate shunt. J Biol Chem. 1984 Aug 10;259(15):9646–9654. [PubMed] [Google Scholar]


