Abstract
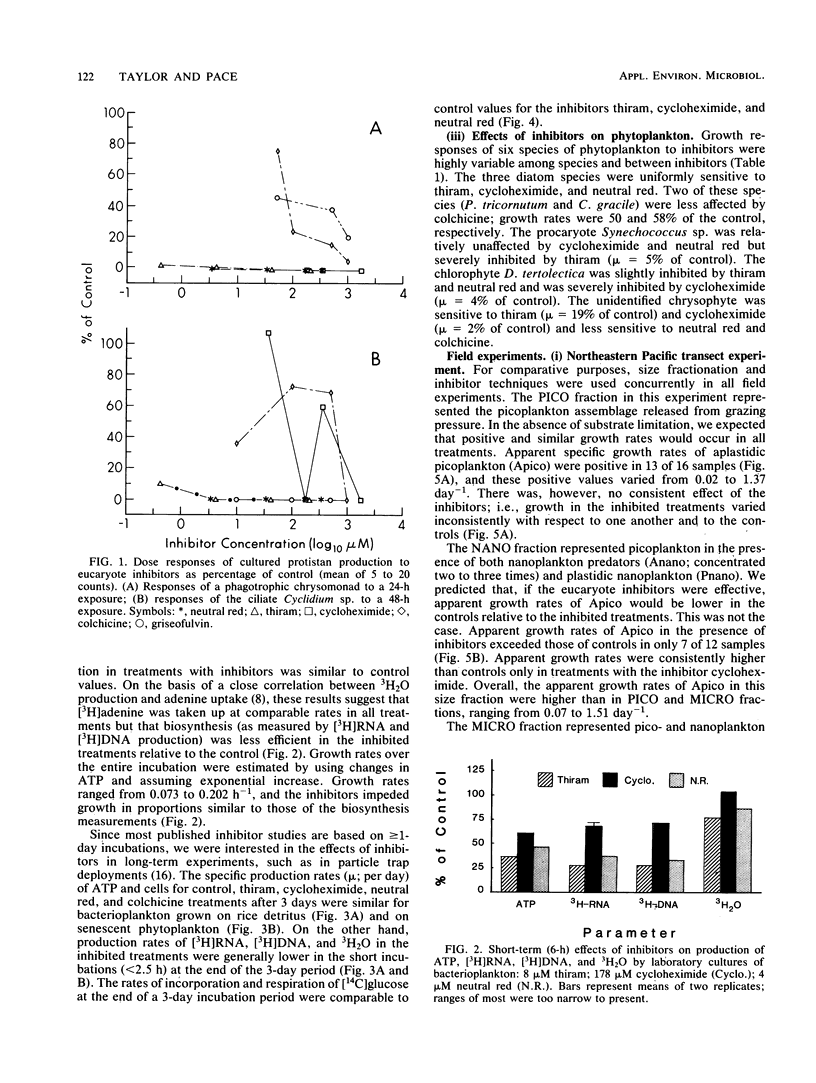
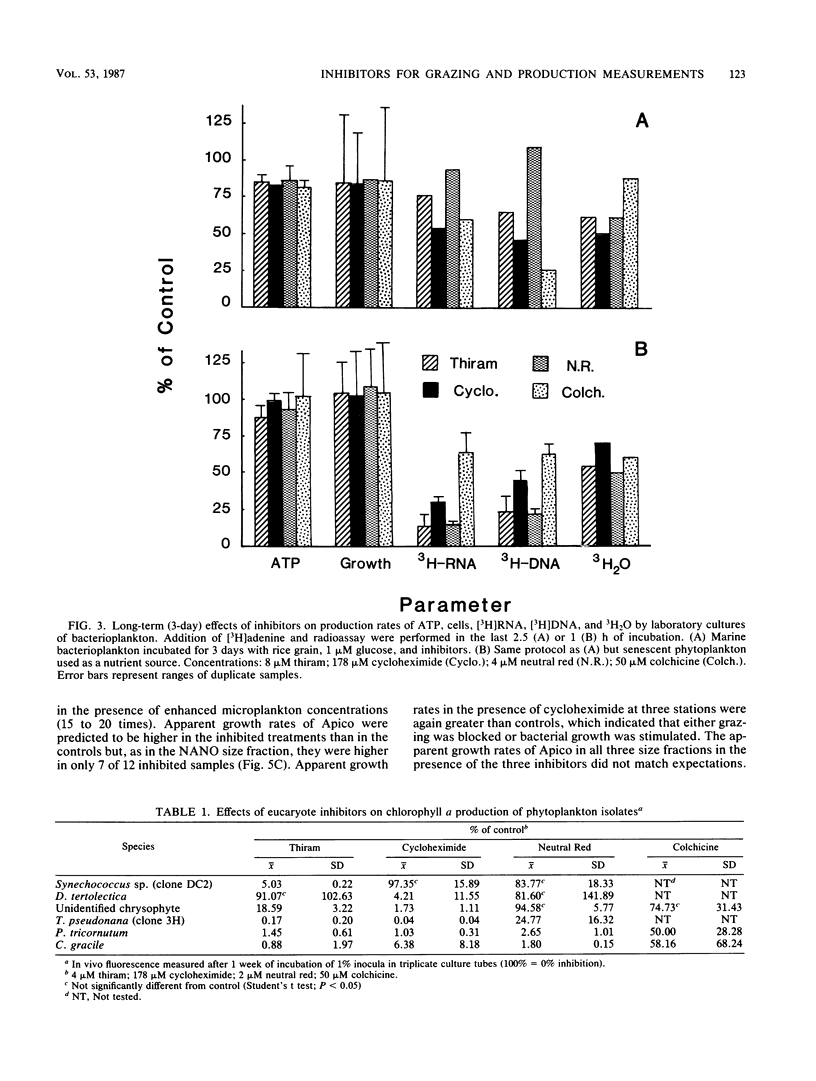
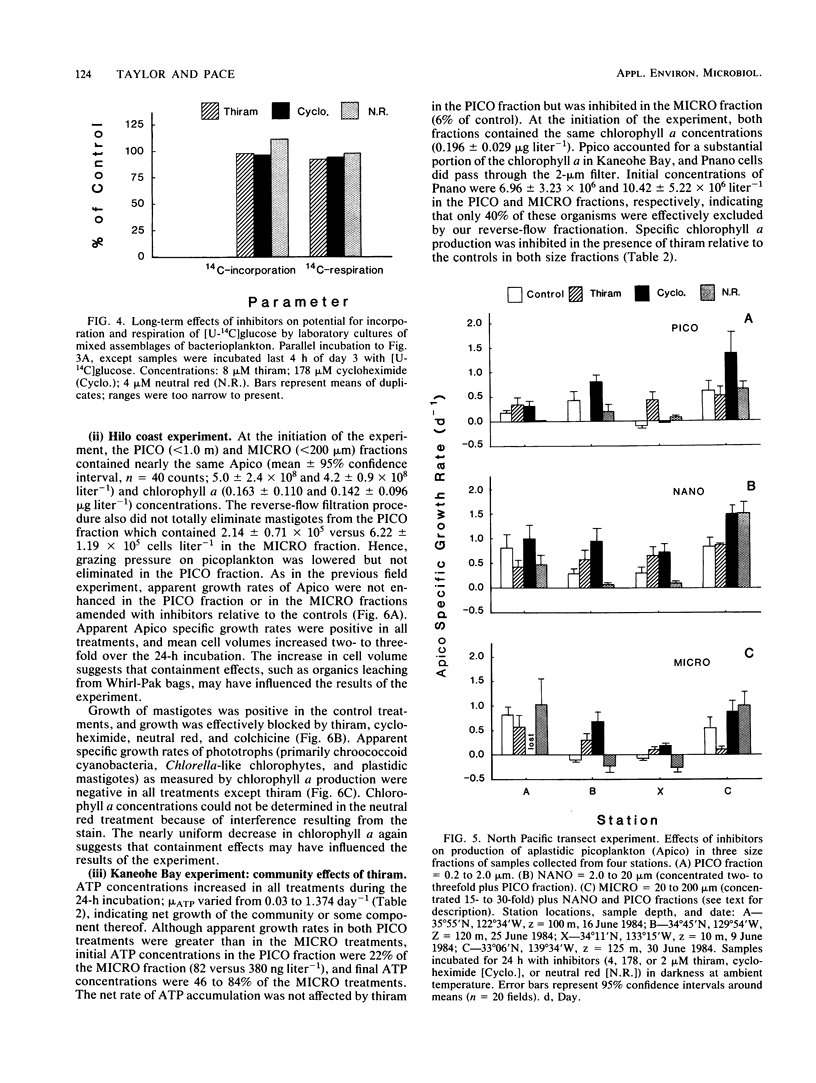
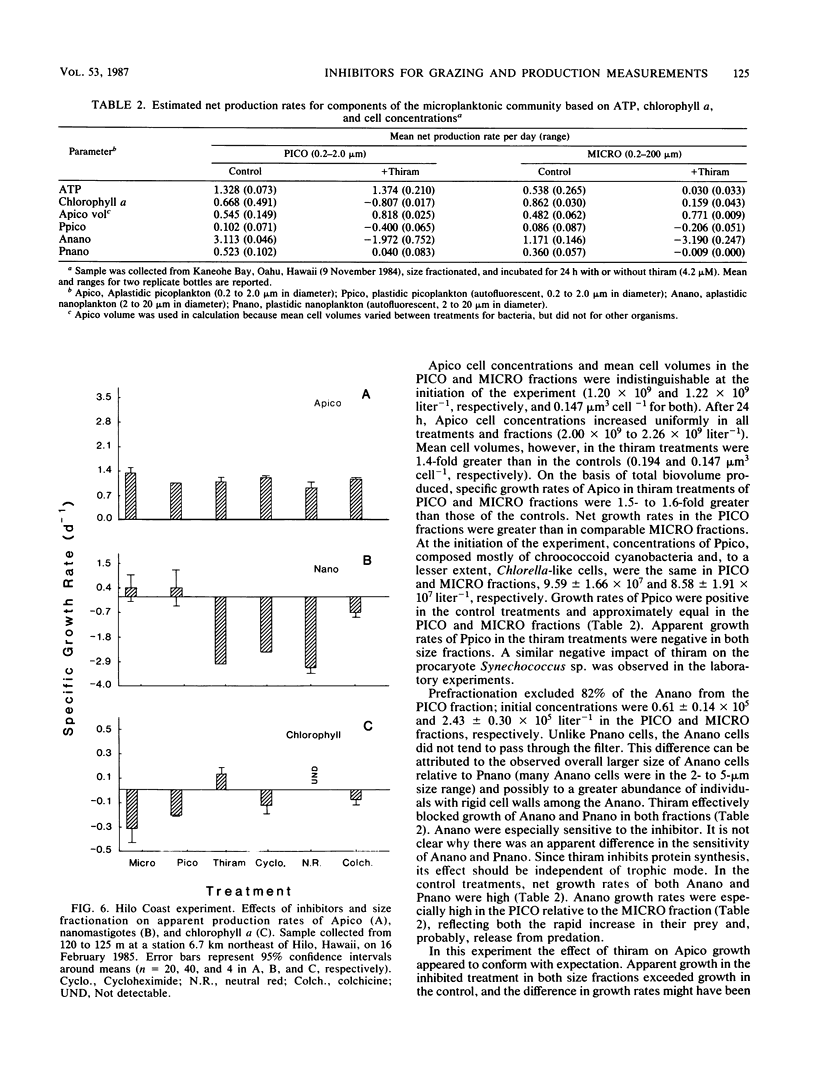
Application of eucaryote inhibitors to the estimation of production and grazing mortality of bacterioplankton was evaluated. Exposure to a range of concentrations of thiram, cycloheximide, and neutral red (0.4 to 210, 36 to 1,777, 4 to 346 μM, respectively) was 98 to 100% effective at inhibiting growth of a chrysomonad in culture. Exposure to colchicine and griseofulvin (50 to 1,000 μM for both) yielded only 24 to 94 and 53 to 79% inhibition, respectively. Exposures to thiram, neutral red, and griseofulvin were 90 to 100% effective at inhibiting growth in culture of a ciliate, Cyclidium sp., and the responses to colchicine and cycloheximide were variable (64 to 100 and 0 to 100% inhibition, respectively). Thiram and neutral red inhibited field populations of nanozooplankton more effectively than cycloheximide and colchicine. Direct effects of eucaryote inhibitors on growing cultures of bacterioplankton varied with parameters measured and duration of exposure. After 3-day exposures, specific growth rates and “instantaneous” heterotrophic potential ([14C]glucose uptake) were not consistently affected, but biosynthetic activity (RNA and DNA syntheses) was depressed. The degree of inhibition of isolates and field populations of phytoplankton depended upon type of inhibitor and phytoplankton species. In field experiments, it was possible to calculate rates of bacterioplankton production and grazing mortality for only 16 of 29 inhibitor experiments and for 4 of 10 size fractionation experiments. Bacterioplankton production and mortality estimates varied greatly with the eucaryote inhibitor used, and those derived from inhibition techniques were substantially different from those derived from fractionation techniques. The poor performances of both techniques are attributed to the following: (i) effects of inhibitors on phytoplankton, (ii) indirect effects of the inhibitors on bacterioplankton, and (iii) insufficient separation of grazers from prey by filtration techniques. Because of the inconsistent results obtained in this investigation, we strongly recommend exercising caution in the application of inhibitor techniques to ecological problems, especially in phototrophically dominated systems.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., McManus G. B. Do bacteria-sized marine eukaryotes consume significant bacterial production? Science. 1984 Jun 15;224(4654):1257–1260. doi: 10.1126/science.224.4654.1257. [DOI] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Simultaneous rates of ribonucleic Acid and deoxyribonucleic Acid syntheses for estimating growth and cell division of aquatic microbial communities. Appl Environ Microbiol. 1981 Nov;42(5):802–810. doi: 10.1128/aem.42.5.802-810.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. W., Porter K. G. Use of metabolic inhibitors to estimate protozooplankton grazing and bacterial production in a monomictic eutrophic lake with an anaerobic hypolimnion. Appl Environ Microbiol. 1986 Jul;52(1):101–107. doi: 10.1128/aem.52.1.101-107.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


