Abstract
Naphthalene biodegradation was investigated in microcosms containing sediment and water collected from three ecosystems which varied in past exposure to anthropogenic and petrogenic chemicals. Mineralization half-lives for naphthalene in microcosms ranged from 2.4 weeks in sediment chronically exposed to petroleum hydrocarbons to 4.4 weeks in sediment from a pristine environment. Microbiological analysis of sediments indicated that hydrocarbon-utilizing microbial populations also varied among ecosystems and were 5 to 12 times greater in sediment after chronic petrogenic chemical exposure than in sediment from an uncontaminated ecosystem. Sediment from an ecosystem exposed to agricultural chemicals had a mineralization half-life of 3.2 weeks for naphthalene and showed about a 30-fold increase in heterotrophic bacterial populations in comparison to uncontaminated sediments, but only a 2- to 3-fold increase in hydrocarbon-degrading bacteria. Analysis of organic solvent-extractable residues from the microcosms by high-pressure liquid chromatography detected polar metabolites which accounted for 1 to 3% of the total radioactivity. Purification of these residues by thin-layer chromatography and further analysis by gas chromatography-mass spectrometry indicated that cis-1,2-dihydroxy-1,2-dihydronaphthalene, 1-naphthol, salicylic acid, and catechol were metabolites of naphthalene. These results provide useful estimates for the rates of naphthalene mineralization in different natural ecosystems and on the degradative pathway for microbial metabolism of naphthalene in freshwater and estuarine environments.
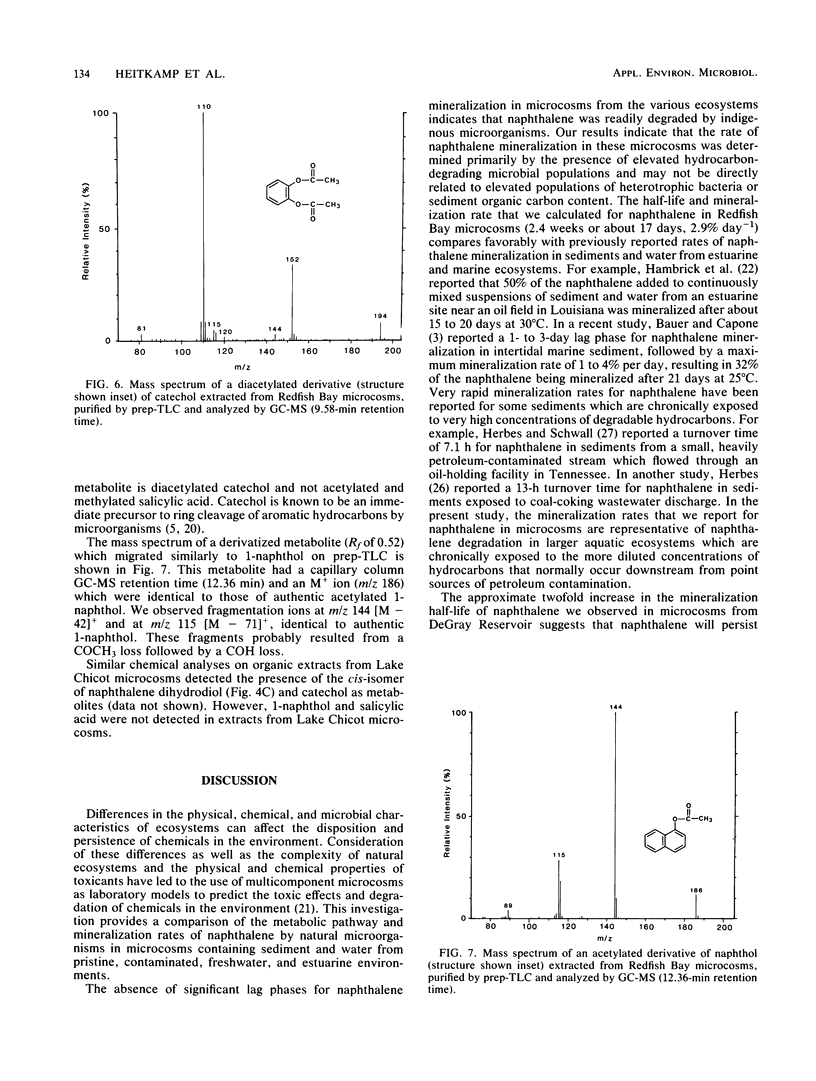
Full text
PDF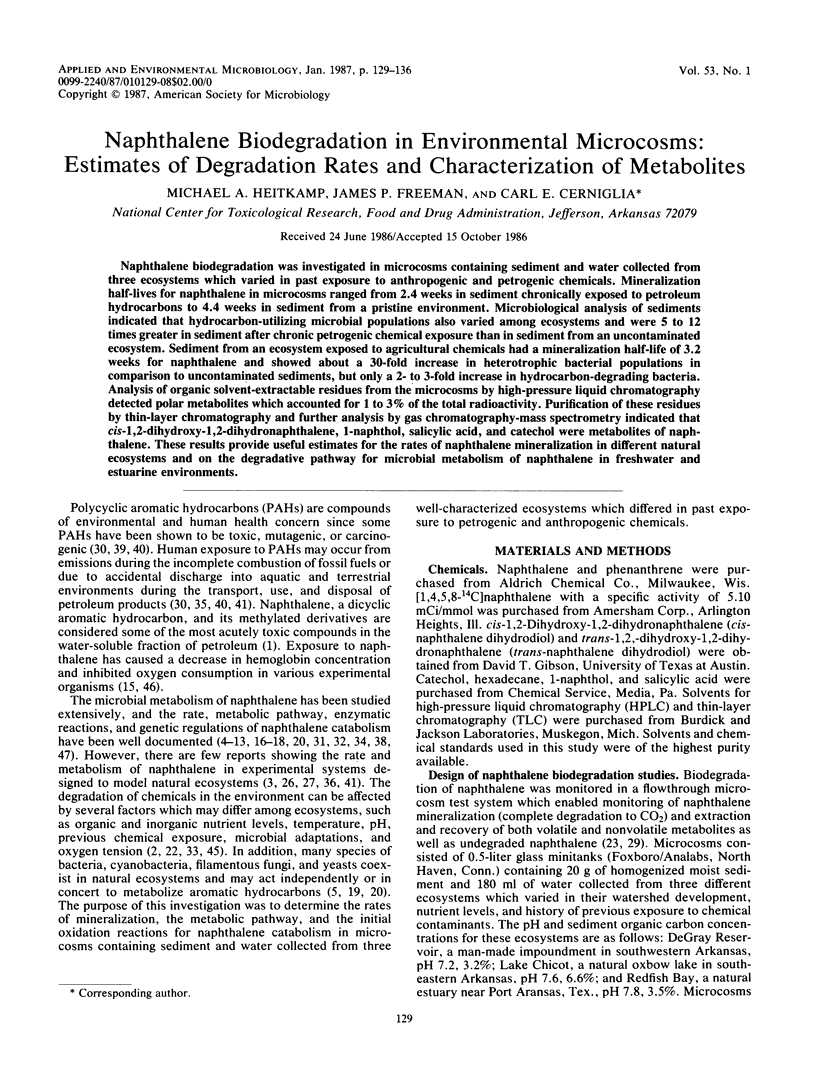



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas R. M. Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev. 1981 Mar;45(1):180–209. doi: 10.1128/mr.45.1.180-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J. E., Capone D. G. Degradation and mineralization of the polycyclic aromatic hydrocarbons anthracene and naphthalene in intertidal marine sediments. Appl Environ Microbiol. 1985 Jul;50(1):81–90. doi: 10.1128/aem.50.1.81-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS V. G., WILLOUGHBY L. G. The distribution of bacteria and fungal spores in Blelham Tarn with particular reference to an experimental overturn. Arch Mikrobiol. 1962;43:294–307. doi: 10.1007/BF00405972. [DOI] [PubMed] [Google Scholar]
- Catterall F. A., Murray K., Williams P. A. The configuration of the 1,2-dihydroxy-1,2-dihydronaphthalene formed in the bacterial metabolism of naphthalene. Biochim Biophys Acta. 1971 May 18;237(2):361–364. doi: 10.1016/0304-4165(71)90331-x. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Althaus J. R., Evans F. E., Freeman J. P., Mitchum R. K., Yang S. K. Stereochemistry and evidence for an arene oxide-NIH shift pathway in the fungal metabolism of naphthalene. Chem Biol Interact. 1983 Apr-May;44(1-2):119–132. doi: 10.1016/0009-2797(83)90134-5. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Freeman J. P., Evans F. E. Evidence for an arene oxide-NIH shift pathway in the transformation of naphthalene to 1-naphthol by Bacillus cereus. Arch Microbiol. 1984 Aug;138(4):283–286. doi: 10.1007/BF00410891. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Freeman J. P., Mitchum R. K. Glucuronide and sulfate conjugation in the fungal metabolism of aromatic hydrocarbons. Appl Environ Microbiol. 1982 May;43(5):1070–1075. doi: 10.1128/aem.43.5.1070-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Gibson D. T. Metabolism of naphthalene by Cunninghamella elegans. Appl Environ Microbiol. 1977 Oct;34(4):363–370. doi: 10.1128/aem.34.4.363-370.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Gibson D. T. Metabolism of naphthalene by cell extracts of Cunninghamella elegans. Arch Biochem Biophys. 1978 Feb;186(1):121–127. doi: 10.1016/0003-9861(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Hebert R. L., Szaniszlo P. J., Gibson D. T. Fungal transformation of naphthalene. Arch Microbiol. 1978 May 30;117(2):135–143. doi: 10.1007/BF00402301. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv Appl Microbiol. 1984;30:31–71. doi: 10.1016/s0065-2164(08)70052-2. [DOI] [PubMed] [Google Scholar]
- Davies J. I., Evans W. C. Oxidative metabolism of naphthalene by soil pseudomonads. The ring-fission mechanism. Biochem J. 1964 May;91(2):251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T., Laborde A. L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982 Mar;149(3):948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T. Naphthalene dioxygenase: purification and properties of a terminal oxygenase component. J Bacteriol. 1983 Aug;155(2):505–511. doi: 10.1128/jb.155.2.505-511.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrick G. A., Delaune R. D., Patrick W. H. Effect of Estuarine Sediment pH and Oxidation-Reduction Potential on Microbial Hydrocarbon Degradation. Appl Environ Microbiol. 1980 Aug;40(2):365–369. doi: 10.1128/aem.40.2.365-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp M. A., Freeman J. P., Cerniglia C. E. Biodegradation of tert-butylphenyl diphenyl phosphate. Appl Environ Microbiol. 1986 Feb;51(2):316–322. doi: 10.1128/aem.51.2.316-322.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp M. A., Johnson B. T. Impact of an oil field effluent on microbial activities in a Wyoming river. Can J Microbiol. 1984 Jun;30(6):786–792. doi: 10.1139/m84-120. [DOI] [PubMed] [Google Scholar]
- Herbes S. E. Rates of microbial transformation of polycyclic aromatic hydrocarbons in water and sediments in the vicinity of a coal-coking wastewater discharge. Appl Environ Microbiol. 1981 Jan;41(1):20–28. doi: 10.1128/aem.41.1.20-28.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbes S. E., Schwall L. R. Microbial transformation of polycyclic aromatic hydrocarbons in pristine and petroleum-contaminated sediments. Appl Environ Microbiol. 1978 Feb;35(2):306–316. doi: 10.1128/aem.35.2.306-316.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder C. L., Korfmacher W. A., Slikker W., Jr, Thompson H. C., Jr, Gosnell A. B. Mass spectral characterization of doxylamine and its rhesus monkey urinary metabolites. Biomed Mass Spectrom. 1985 Apr;12(4):151–158. doi: 10.1002/bms.1200120403. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Yeh H. J., Jerina D. M., Patel T. R., Davey J. F., Gibson D. T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975 Feb 11;14(3):575–584. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Jeffrey A. M., Gibson D. T. Cis-1,2-dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971 Jan;142(1):394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- Rowe R., Todd R., Waide J. Microtechnique for most-probable-number analysis. Appl Environ Microbiol. 1977 Mar;33(3):675–680. doi: 10.1128/aem.33.3.675-680.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushing L. G., Althaus J. R., Thompson H. C., Jr Simultaneous determination of trimellitic anhydride and its trimellitic acid impurity by GC/FID. J Anal Toxicol. 1982 Nov-Dec;6(6):290–293. doi: 10.1093/jat/6.6.290. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Pritchard P. H., Bourquin A. W. Effects of adaptation on biodegradation rates in sediment/water cores from estuarine and freshwater environments. Appl Environ Microbiol. 1980 Oct;40(4):726–734. doi: 10.1128/aem.40.4.726-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struble V. G., Harmon H. J. Molecular basis for inhibition of mitochondrial respiration by naphthalene. Bull Environ Contam Toxicol. 1983 Dec;31(6):644–648. doi: 10.1007/BF01606040. [DOI] [PubMed] [Google Scholar]
- TRECCANI V., WALKER N., WILTSHIRE G. H. The metabolism of naphthalene by soil bacteria. J Gen Microbiol. 1954 Dec;11(3):341–348. doi: 10.1099/00221287-11-3-341. [DOI] [PubMed] [Google Scholar]
- Turner B. C., Glotfelty D. E. Field air sampling of pesticide vapors with polyurethane foam. Anal Chem. 1977 Jan;49(1):7–10. doi: 10.1021/ac50009a009. [DOI] [PubMed] [Google Scholar]


