Abstract
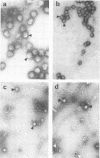
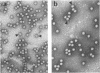
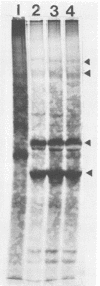
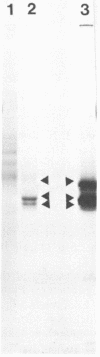
Representatives of several families of insect viruses were tested for growth and pathogenicity in the olive fruit fly, Dacus oleae Gmel. The viruses included nuclear polyhedrosis viruses, an iridovirus, two picornaviruses, and Trichoplusia ni small RNA virus (a member of the Nudaurelia β family), in addition to two naturally occurring viruses of the olive fruit fly. Two viruses, one of the two picornaviruses (cricket paralysis virus [CrPV] and the iridovirus (type 21 from Heliothis armigera), were found to replicate in adult flies. Flies which were fed on a solution containing CrPV for 1 day demonstrated a high mortality with 50% dying within 5 days and nearly 80% dying within 12 days of being fed. The virus was transmissible from infected to noninfected flies by fecal contamination. The CrPV which replicated in the infected flies was demonstrated to be the same as input virus by infection of Drosophila melanogaster cells and examination of the expressed viral proteins, immunoprecipitation of the virus purified from flies, and electrophoretic analysis of the structural proteins.
Full text
PDF
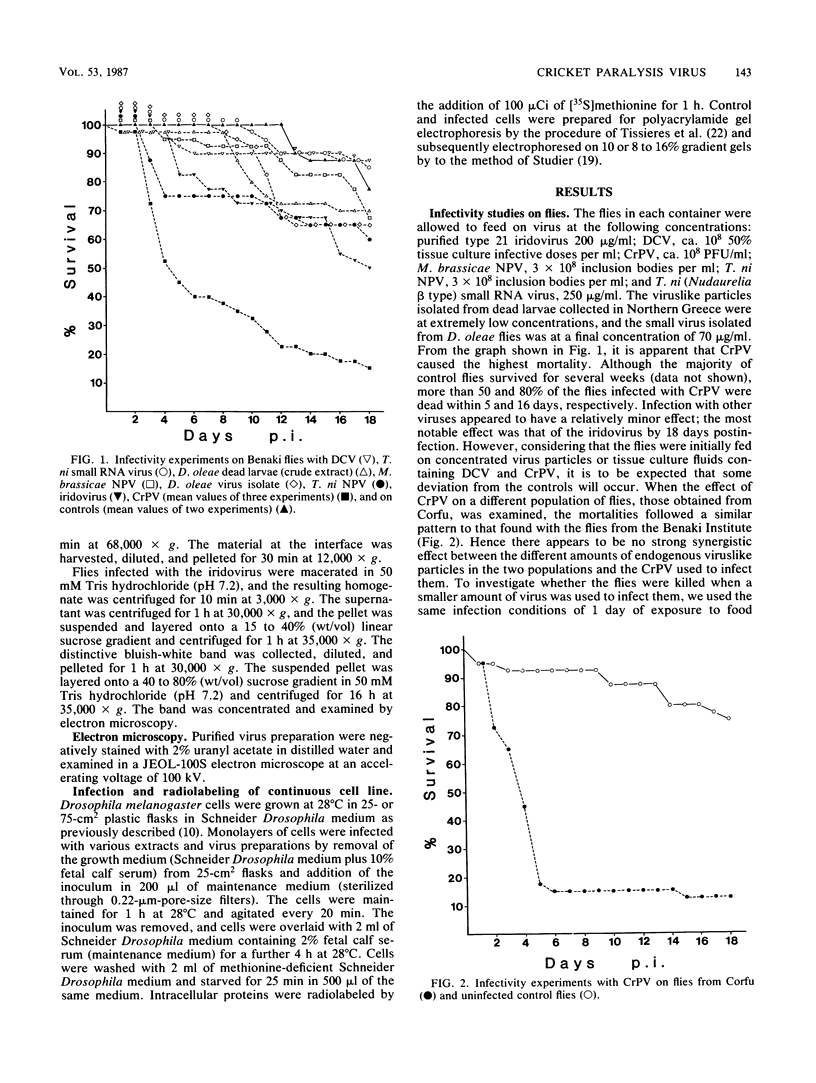


Images in this article
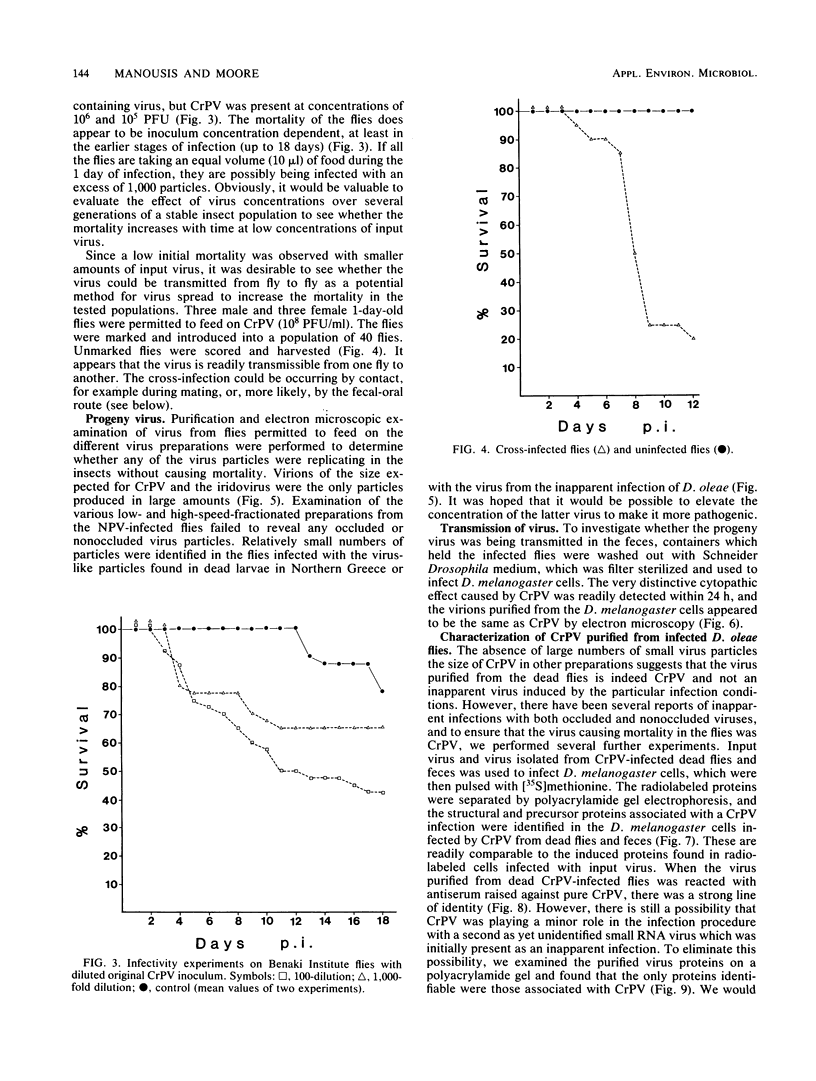
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. A., Evans H. F., Allen C. J., Kelly D. C. Biological and biochemical investigations on five European isolates of Mamestra brassica nuclear polyhedrosis virus. Arch Virol. 1981;69(3-4):209–217. doi: 10.1007/BF01317336. [DOI] [PubMed] [Google Scholar]
- Carey G. P., Lescott T., Robertson J. S., Spencer L. K., Kelly D. C. Three African isolates of small iridescent viruses: type 21 from Heliothis armigera (Lepidoptera: Noctuidae), type 23 from Heteronychus arator (Coleoptera: Scarabaeidae), and type 28 from Lethocerus columbiae (Hemiptera Heteroptera: Belostomatidae). Virology. 1978 Mar;85(1):307–309. doi: 10.1016/0042-6822(78)90434-8. [DOI] [PubMed] [Google Scholar]
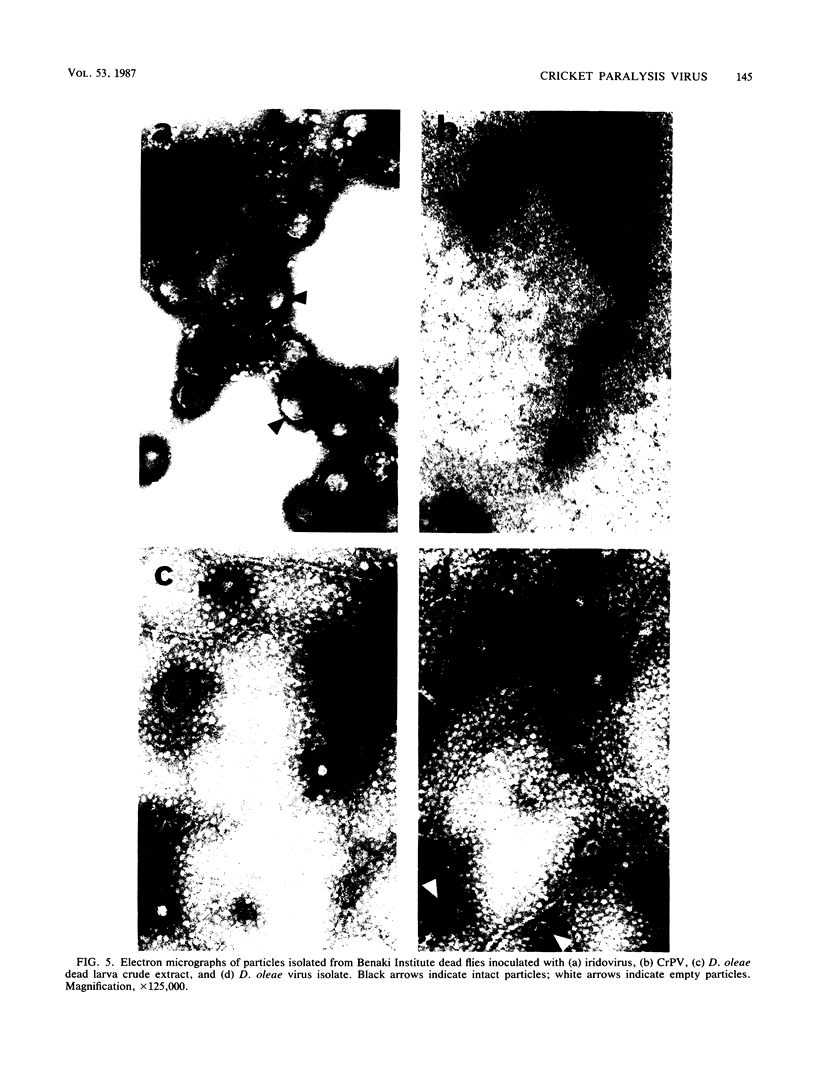
- Harrap K. A. Assessment of the human and ecological hazards of microbial insecticides. Parasitology. 1982 Apr;84(Pt 4):269–296. doi: 10.1017/s0031182000053671. [DOI] [PubMed] [Google Scholar]
- Harrap K. A., Longworth J. F., Tinsley T. W. A noninclusion virus of Gonometa podocarpi (Lepidoptera: Lasiocampidae). J Invertebr Pathol. 1966 Jun;8(2):270–272. doi: 10.1016/0022-2011(66)90147-9. [DOI] [PubMed] [Google Scholar]
- Moore N. F., Kearns A., Pullin J. S. Characterization of cricket paralysis virus-induced polypeptides in Drosophila cells. J Virol. 1980 Jan;33(1):1–9. doi: 10.1128/jvi.33.1.1-9.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinganum C. The isolation of cricket paralysis virus from the emperor gum moth, Antheraea eucalypti Scott, and its infectivity towards a range of insect species. Intervirology. 1975;5(1-2):97–102. doi: 10.1159/000149886. [DOI] [PubMed] [Google Scholar]
- Scotti P. D., Longworth J. F. Naturally occurring IgM antibodies to a small RNA insect virus in some mammalian sera in New Zealand. Intervirology. 1980;13(3):186–191. doi: 10.1159/000149124. [DOI] [PubMed] [Google Scholar]
- Scotti P. D., Longworth J. F., Plus N., Croizier G., Reinganum C. The biology and ecology of strains of an insect small RNA virus complex. Adv Virus Res. 1981;26:117–143. doi: 10.1016/s0065-3527(08)60422-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tinsley T. W., MacCallum F. O., Robertson J. S., Brown F. Relationship of encephalomyocarditis virus to cricket paralysis virus of insects. Intervirology. 1984;21(4):181–186. doi: 10.1159/000149519. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]