Abstract
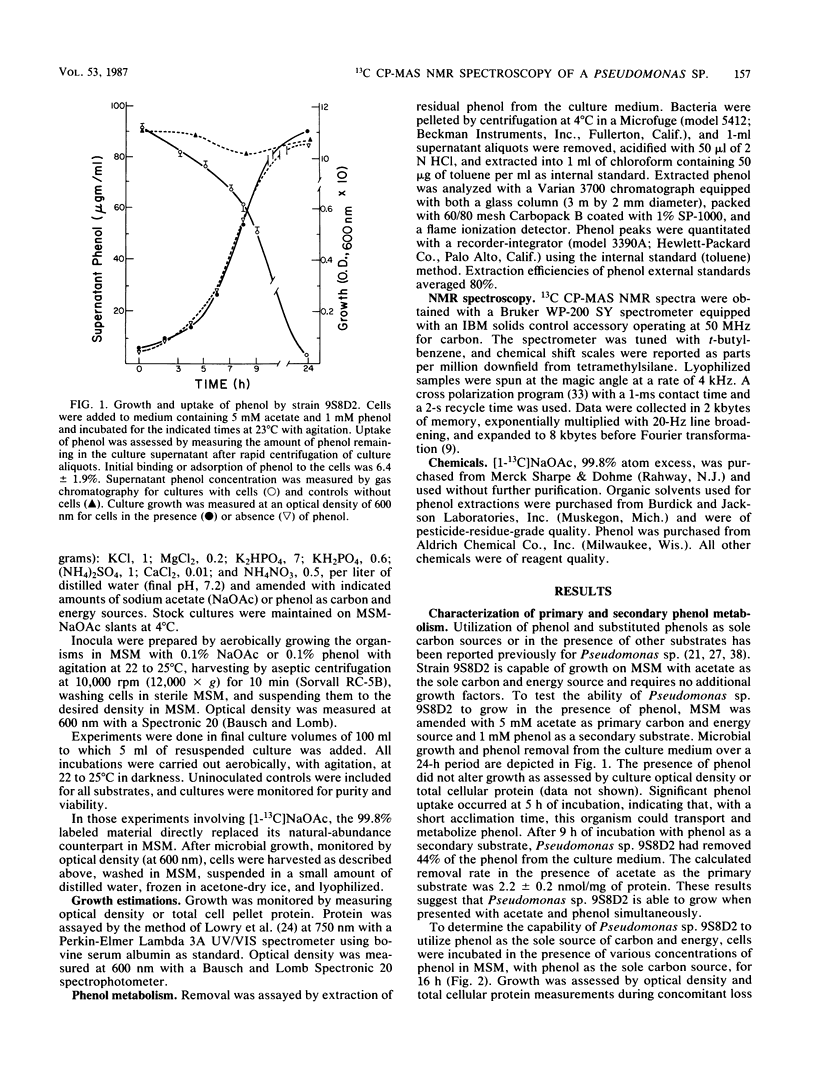
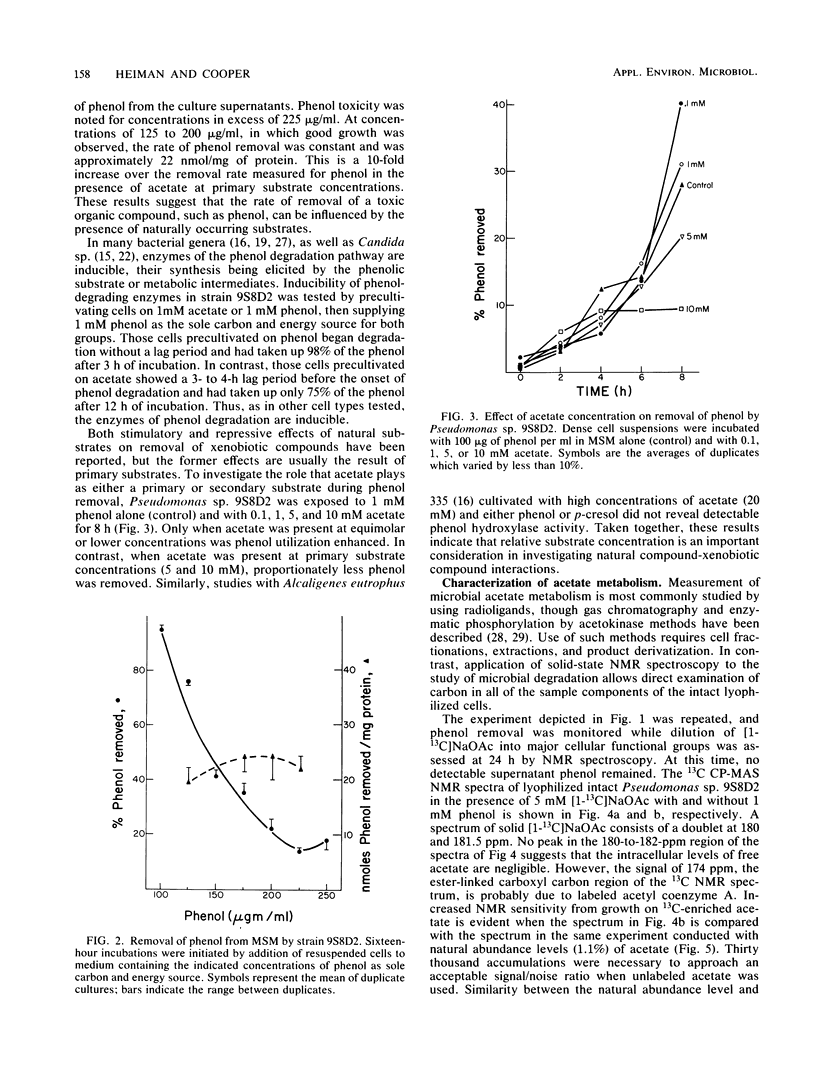
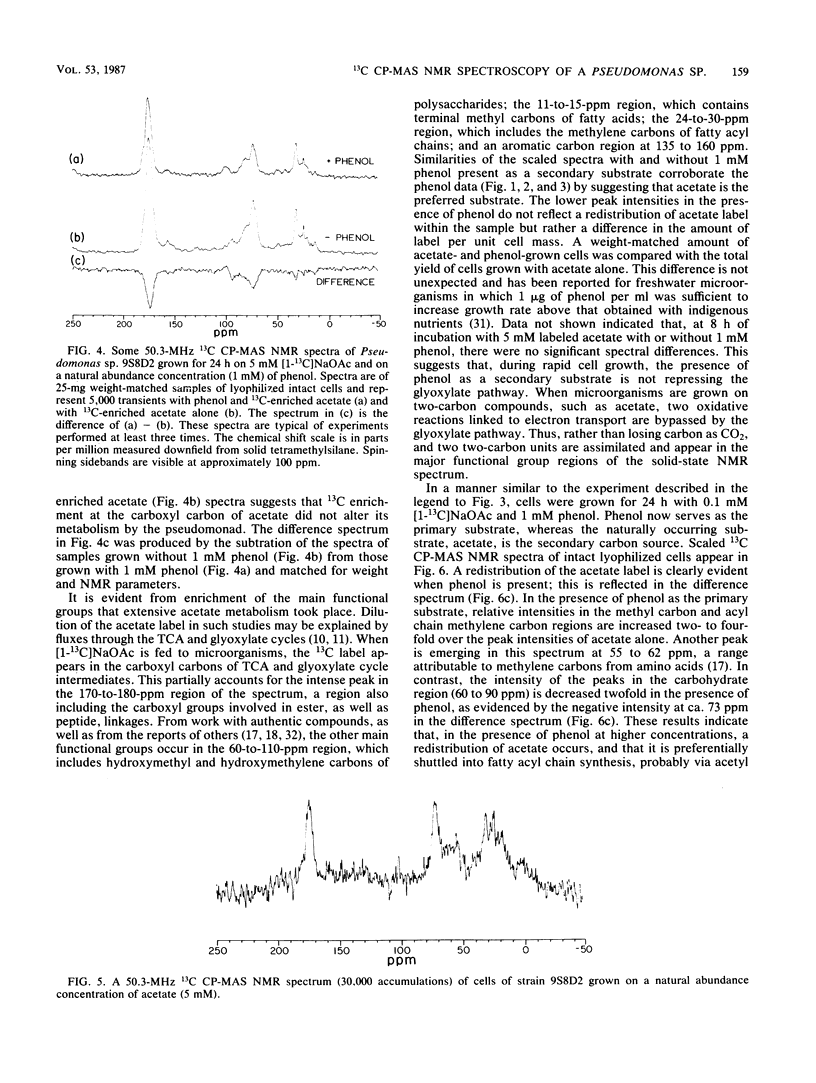
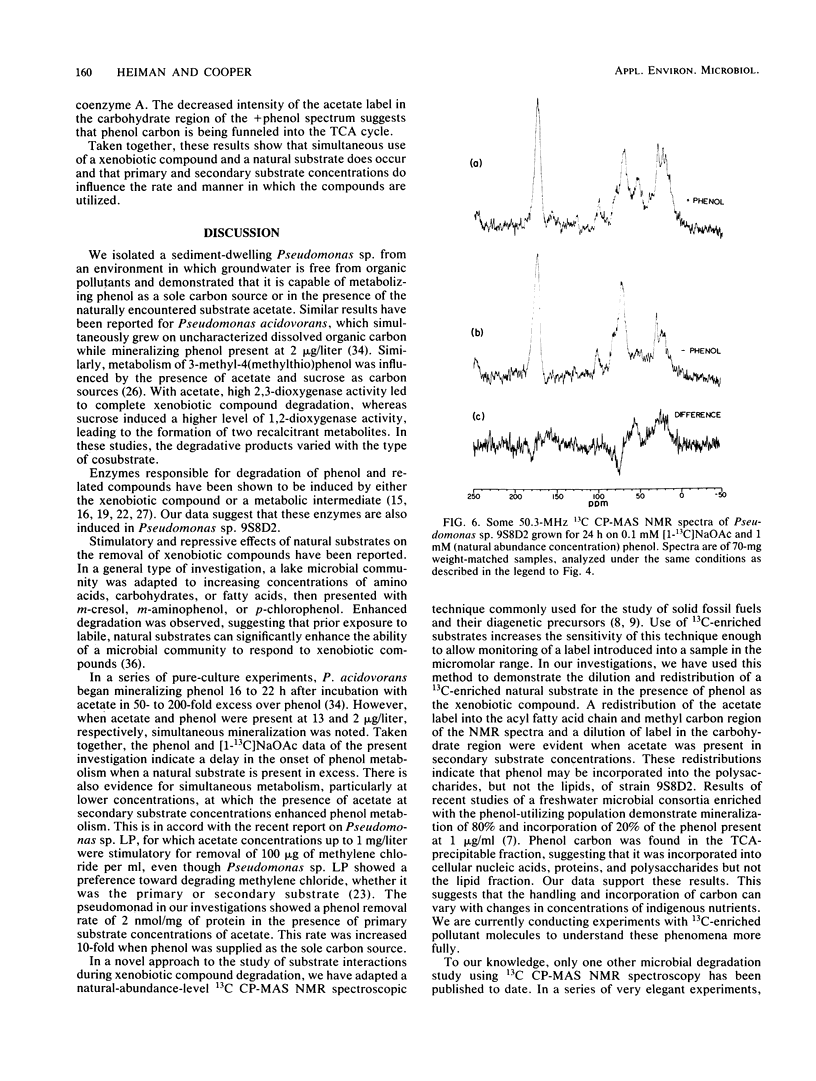
We investigated concentration-dependent primary and secondary substrate relationships in the simultaneous metabolism of the ubiquitous pollutant phenol and the naturally occurring substrate acetate by a Pseudomonas sp. soil isolate capable of utilizing either substance as a sole source of carbon and energy. In addition to conventional analytical techniques, solid-state 13C nuclear magnetic resonance spectroscopy was used to follow the cellular distribution of [1-13C]acetate in the presence of unlabeled phenol. With 5 mM acetate as the primary substrate, Pseudomonas sp. 9S8D2 removed 1 mM phenol (secondary substrate) at a rate of 2 nmol/mg of total cell protein. Although extensive acetate metabolism was indicated by a significant redistribution of the carboxyl label, this redistribution was not affected by the presence of phenol as a secondary substrate. When the primary and secondary substrate roles were reversed, however, the presence of 1 mM phenol altered the metabolism of 0.1 mM acetate, as evidenced by both the two- to fourfold increases in carboxyl label that appeared in terminal methyl and acyl chain methylene carbon resonances and the decrease in label that occurred in the carbohydrate spectral region. These results suggest that, when phenol is present as the primary substrate, acetate is preferentially shuttled into fatty acyl chain synthesis, whereas phenol carbon is funnelled into the tricarboxylic acid cycle. Thus, simultaneous use of a xenobiotic compound and a natural substrate apparently does occur, and the relative concentrations of the two substrates do influence the rate and manner in which the compounds are utilized.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation of chemicals of environmental concern. Science. 1981 Jan 9;211(4478):132–138. doi: 10.1126/science.7444456. [DOI] [PubMed] [Google Scholar]
- Balkwill D. L., Ghiorse W. C. Characterization of subsurface bacteria associated with two shallow aquifers in oklahoma. Appl Environ Microbiol. 1985 Sep;50(3):580–588. doi: 10.1128/aem.50.3.580-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J. E., Capone D. G. Effects of four aromatic organic pollutants on microbial glucose metabolism and thymidine incorporation in marine sediments. Appl Environ Microbiol. 1985 Apr;49(4):828–835. doi: 10.1128/aem.49.4.828-835.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R. L. Microbiological applications of NMR spectroscopy. Part 1. Microbiol Sci. 1985 Jul;2(7):203-6, 211. [PubMed] [Google Scholar]
- Baxter R. L. Microbiological applications of NMR spectroscopy. Part 2. Microbiol Sci. 1985 Nov;2(11):340–345. [PubMed] [Google Scholar]
- Brilon C., Beckmann W., Knackmuss H. J. Catabolism of Naphthalenesulfonic Acids by Pseudomonas sp. A3 and Pseudomonas sp. C22. Appl Environ Microbiol. 1981 Jul;42(1):44–55. doi: 10.1128/aem.42.1.44-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney R. H., Sollitti P., Rubin H. E. Incorporation of phenol carbon at trace concentrations by phenol-mineralizing microorganisms in fresh water. Appl Environ Microbiol. 1985 Jan;49(1):15–18. doi: 10.1128/aem.49.1.15-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp M. A., Freeman J. P., Cerniglia C. E. Biodegradation of tert-butylphenyl diphenyl phosphate. Appl Environ Microbiol. 1986 Feb;51(2):316–322. doi: 10.1128/aem.51.2.316-322.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E. J., Bayly R. C. Control of catechol meta-cleavage pathway in Alcaligenes eutrophus. J Bacteriol. 1983 Jun;154(3):1363–1370. doi: 10.1128/jb.154.3.1363-1370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob G. S., Schaefer J., Stejskal E. O., McKay R. A. Solid-state NMR determination of glyphosate metabolism in a Pseudomonas sp. J Biol Chem. 1985 May 25;260(10):5899–5905. [PubMed] [Google Scholar]
- Jacob G. S., Schaefer J., Wilson G. E., Jr Solid-state 13C and 15N nuclear magnetic resonance studies of alanine metabolism in Aerococcus viridans (Gaffkya homari). J Biol Chem. 1985 Mar 10;260(5):2777–2781. [PubMed] [Google Scholar]
- Johnson B. F., Stanier R. Y. Regulation of the -ketoadipate pathway in Alcaligenes eutrophus. J Bacteriol. 1971 Aug;107(2):476–485. doi: 10.1128/jb.107.2.476-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackmuss H. J., Hellwig M. Utilization and cooxidation of chlorinated phenols by Pseudomonas sp. B 13. Arch Microbiol. 1978 Apr 27;117(1):1–7. doi: 10.1007/BF00689343. [DOI] [PubMed] [Google Scholar]
- Krug M., Ziegler H., Straube G. Degradation of phenolic compounds by the yeast Candida tropicalis HP 15. I. Physiology of growth and substrate utilization. J Basic Microbiol. 1985;25(2):103–110. doi: 10.1002/jobm.3620250206. [DOI] [PubMed] [Google Scholar]
- LaPat-Polasko L. T., McCarty P. L., Zehnder A. J. Secondary substrate utilization of methylene chloride by an isolated strain of Pseudomonas sp. Appl Environ Microbiol. 1984 Apr;47(4):825–830. doi: 10.1128/aem.47.4.825-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- Rogosa M., Love L. L. Direct quantitative gas chromatographic separation of C2-C6 fatty acids, methanol, and ethyl alcohol in aqueous microbial fermentation media. Appl Microbiol. 1968 Feb;16(2):285–290. doi: 10.1128/am.16.2.285-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. E., Schmidt S. Growth of phenol-mineralizing microorganisms in fresh water. Appl Environ Microbiol. 1985 Jan;49(1):11–14. doi: 10.1128/aem.49.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. K., Alexander M. Effects of dissolved organic carbon and second substrates on the biodegradation of organic compounds at low concentrations. Appl Environ Microbiol. 1985 Apr;49(4):822–827. doi: 10.1128/aem.49.4.822-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela S., Pai S. B. Metabolism of fensulfothion by a soil bacterium, Pseudomonas alcaligenes C1. Appl Environ Microbiol. 1983 Aug;46(2):475–479. doi: 10.1128/aem.46.2.475-479.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R. J., Pfaender F. K. Influence of easily degradable naturally occurring carbon substrates on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol. 1985 Feb;49(2):394–401. doi: 10.1128/aem.49.2.394-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander J. A., Behar K. L., Shulman R. G. 13C NMR study of transamination during acetate utilization by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 May;78(5):2693–2697. doi: 10.1073/pnas.78.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]


