Abstract
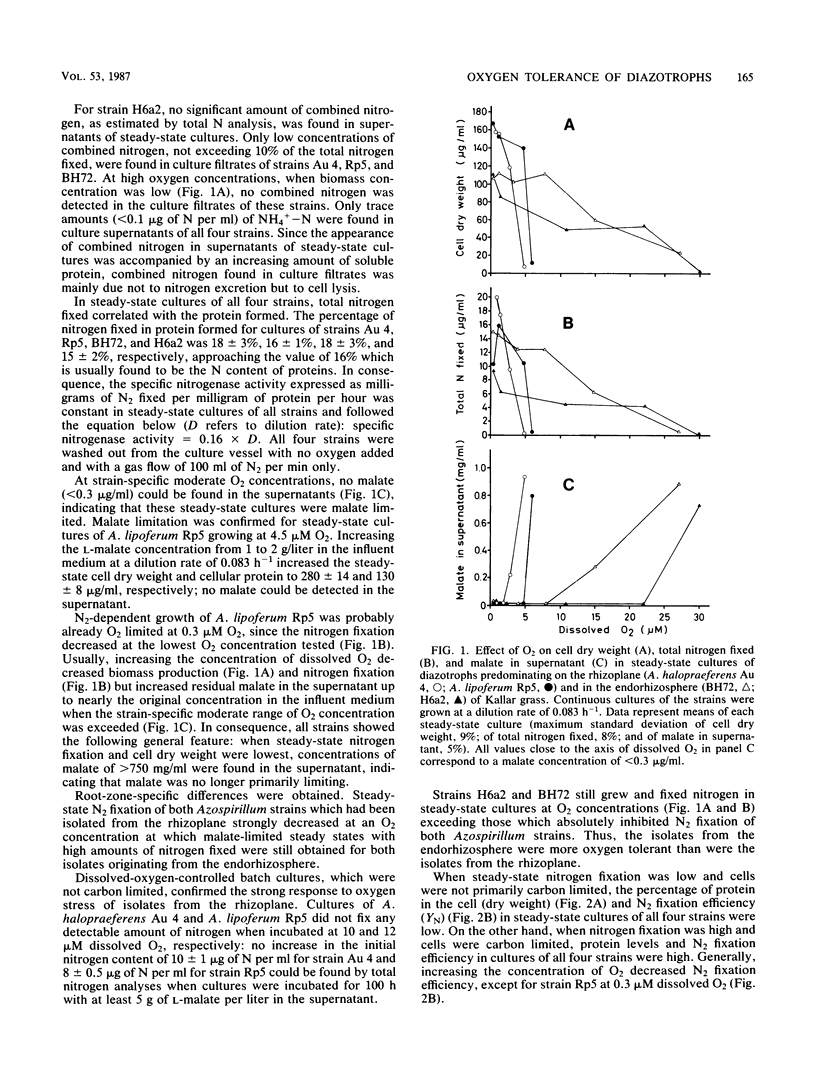
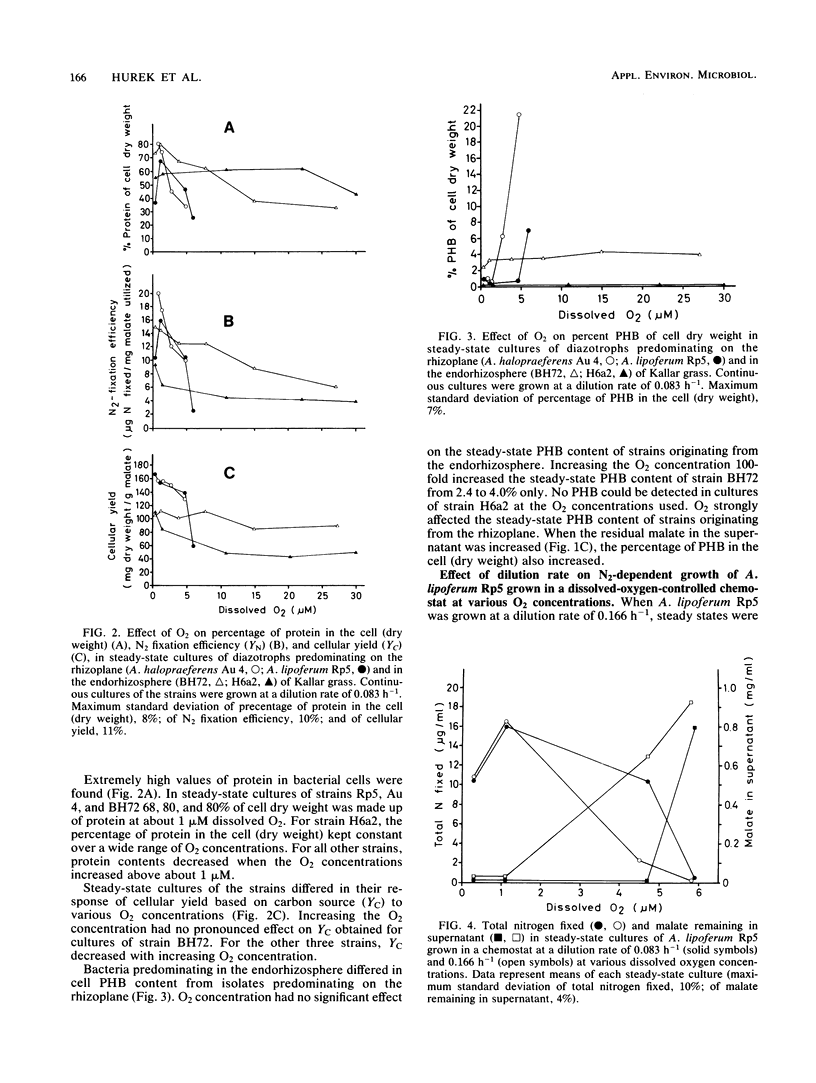
The effect of oxygen on N2-dependent growth of two Azospirillum strains and two diazotrophic rods closely associated with roots of Kallar grass (Leptochloa fusca) was studied. To enable precise comparison, bacteria were grown in dissolved-oxygen-controlled batch and continuous cultures. Steady states were obtained from about 1 to 30 μM O2, some of them being carbon limited. All strains needed a minimum amount of oxygen for N2-dependent growth. Nitrogen contents between 10 and 13% of cell dry weight were observed. The response of steady-state cultures to increasing O2 concentrations suggested that carbon limitation shifted to internal nitrogen limitation when N2 fixation became so low that the bacteria could no longer meet their requirements for fixed nitrogen. For Azospirillum lipoferum Rp5, increase of the dilution rate resulted in decreased N2 fixation in steady-state cultures with internal nitrogen limitation. Oxygen tolerance was found to be strain specific in A. lipoferum with strain Sp59b as a reference organism. Oxygen tolerance of strains from Kallar grass was found to be root zone specific. A. halopraeferens Au 4 and A. lipoferum Rp5, predominating on the rhizoplane of Kallar grass, and strains H6a2 and BH72, predominating in the endorhizosphere, differed in their oxygen tolerance profiles. Strains H6a2 and BH72 still grew and fixed nitrogen in steady-state cultures at O2 concentrations exceeding those which absolutely inhibited nitrogen fixation of both Azospirillum strains. It is proposed that root-zone-specific oxygen tolerance reflects an adaptation of the isolates to the microenvironments provided by the host plant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Turner G. L. Activity of nitrogenase and glutamine synthetase in relation to availability of oxygen in continuous cultures of a strain of cowpea Rhizobium sp. supplied with excess ammonium. Biochim Biophys Acta. 1978 Feb 1;538(3):406–416. doi: 10.1016/0304-4165(78)90402-6. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. The role of O2-limitation in control of nitrogenase in continuous cultures of Rhizobrium sp. Biochem Biophys Res Commun. 1976 Nov 22;73(2):524–531. doi: 10.1016/0006-291x(76)90738-5. [DOI] [PubMed] [Google Scholar]
- Cacciari I., Lippi D., Bordeleau L. M. Effect of oxygen on batch and continuous cultures of a nitrogen-fixing Arthrobacter sp. Can J Microbiol. 1979 Jun;25(6):746–751. doi: 10.1139/m79-108. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- McClung C. R., Patriquin D. G. Isolation of a nitrogen-fixing Campylobacter species from the roots of Spartina alterniflora Loisel. Can J Microbiol. 1980 Aug;26(8):881–886. doi: 10.1139/m80-153. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C. EFFECTS OF ENVIRONMENT ON THE COMPOSITION OF BACTERIAL CELLS. Annu Rev Microbiol. 1963;17:61–86. doi: 10.1146/annurev.mi.17.100163.000425. [DOI] [PubMed] [Google Scholar]
- Nelson L. M., Knowles R. Effect of oxygen and nitrate on nitrogen fixation and denitrification by Azospirillum brasilense grown in continuous culture. Can J Microbiol. 1978 Nov;24(11):1395–1403. doi: 10.1139/m78-223. [DOI] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Factors affecting growth and nitrogen fixation of Spirillum lipoferum. J Bacteriol. 1976 Sep;127(3):1248–1254. doi: 10.1128/jb.127.3.1248-1254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E., Kleiner D., Oelze J. Whole cell respiration and nitrogenase activities in Azotobacter vinelandii growing in oxygen controlled continuous culture. Arch Microbiol. 1983 Jan;134(1):68–72. doi: 10.1007/BF00429410. [DOI] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Fendrik I. Strain-specific chemotaxis of Azospirillum spp. J Bacteriol. 1985 Apr;162(1):190–195. doi: 10.1128/jb.162.1.190-195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Niemann E. G., Fendrik I. Close association of azospirillum and diazotrophic rods with different root zones of kallar grass. Appl Environ Microbiol. 1986 Sep;52(3):520–526. doi: 10.1128/aem.52.3.520-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Senior P. J., Beech G. A., Ritchie G. A., Dawes E. A. The role of oxygen limitation in the formation of poly- -hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem J. 1972 Aug;128(5):1193–1201. doi: 10.1042/bj1281193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. C., Dawes E. A. A disk assay for poly- -hydroxybutyrate. Anal Biochem. 1973 Apr;52(2):607–613. doi: 10.1016/0003-2697(73)90067-5. [DOI] [PubMed] [Google Scholar]
- van Verseveld H. W., Chesbro W. R., Braster M., Stouthamer A. H. Eubacteria have 3 growth modes keyed to nutrient flow. Consequences for the concept of maintenance and maximal growth yield. Arch Microbiol. 1984 Feb;137(2):176–184. doi: 10.1007/BF00414463. [DOI] [PubMed] [Google Scholar]


