Abstract
AIM: To investigate the relationship between low-grade inflammation and several glycemic indices in a population-based sample of men and women. METHODS: The ATTICA study is a population-based cohort that randomly enrolled 1514 men and 1528 women (aged >18 years old), stratified by age and gender, from the Greater Athens area, during 2001-2002. Among several characteristics, inflammation markers (high sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, homocysteine and amyloid A) and glycemic control indices (fasting blood glucose, insulin, HOMA) were measured in the participants. RESULTS: The prevalence of diabetes was 7.8% in men and 6.0% in women. The prevalence of impaired fasting glucose (IFG) was 21% in men and 12% in women. Diabetic subjects had 57% higher mean levels of C-reactive protein (p < 0.001), 22% higher mean levels of interleukin-6 (p < 0.001) and 60% higher levels of tumor necrosis factor-alpha (p < 0.001) compared to non-diabetic subjects. Homocysteine and serum amyloid A levels did not show significant differences among groups. CONCLUSION: Our study supports a positive association between low-grade inflammation and diabetes in a population-based sample of men and women without any evidence of cardiovascular disease, which is independent of demographic, clinical and lifestyle characteristics, including physical activity and dietary factors.
Keywords: type 2 diabetes, C-reactive protein, interleukin-6, TNF-alpha, glycemic control
Introduction
Atherosclerosis is considered in part to be a consequence of chronic low-grade inflammation. Inflammation seems to play a central role in all phases of the atherosclerotic process including plaque initiation, progression and thrombosis [1]. A number of epidemiological studies have demonstrated strong and consistent relationships between sensitive inflammatory markers and subsequent risk of cardiovascular events [2-6]. Insulin resistance and progressive pancreatic beta cell failure are key factors in the development of type 2 diabetes. Several studies have supported the hypothesis that chronic subclinical inflammation may be associated with insulin resistance and precede the development of clinically overt diabetes (type 2) [7-9]. Risk factors for developing diabetes, such as obesity, physical inactivity, smoking, dietary habits, psychological stress and infections, are considered to be activators of the innate immune system that induce a state of chronic low-grade inflammation. Proinflammatory cytokines enhance insulin resistance through molecular pathways that involve activation of Jun kinase (JNK), activation of IκB kinase-β/nuclear factor κB and downregulation of PPARγ expression [8, 24-25]. Pradhan and Ridker [10] have supported the "unifying hypothesis" that both type 2 diabetes and atherosclerosis are multifactorial conditions, which appear to share a common inflammatory basis. Therefore, it seems likely that measures designed to reduce the inflammatory process could be of benefit in reducing the risk of both diabetes and cardiovascular disease. Several studies have associated the consumption of various dietary components with sensitive inflammatory markers, implying that the type and quality of diet may have a significant impact on the inflammatory process [11-13]. In favor of this hypothesis, Chrysohoou et al. reported that people who are closer to the Mediterranean diet have lower levels of several inflammatory markers [14]. The exact association between physical activity and inflammation is not known, however. Some studies have revealed an inverse relationship between physical activity and certain inflammatory markers [15-17], whilst others have not confirmed this association [18].
The aim of this work was to investigate the relationship between low-grade inflammation and the presence of type 2 diabetes, as well as the association between inflammatory marker levels and several glycemic control indices (i.e. blood glucose, insulin levels), in relation to dietary habits and physical activity status, in a population-based sample of men and women from the ATTICA Study [22].
Materials and methods
Study design
The "ATTICA" epidemiological study was carried out in the province of Attica, from May 2001 to December 2002 and finally enrolled 3042 subjects (75% participation rate), of whom 1514 were men and 1528 were women. The sampling was random, anticipated enrolling included only one participant per household and all people living in institutions were excluded. 5% of men and 3% of women were excluded because they reported a history of cardiovascular or another atherosclerotic disease, as well as chronic viral infections. The number of enrolled participants was adequate to evaluate two-tailed hypotheses regarding differences in investigated parameters between the subgroups in the study greater than 0.5 standard deviations, achieving statistical power greater than 0.90 at 5% probability level (p-value).
Further details regarding the design and methodology of the ATTICA study have been previously presented [22].
Investigated parameters
The evaluation of nutritional habits was based on the guidelines from the Department of Nutrition of the National School of Public Health [19]. Consumption of non-refined cereals and products, vegetables, legumes, fruits, olive oil, dairy products, fish, pulses, nuts, potatoes, eggs, sweets, poultry, red meat and meat products was measured as an average per week during the past year using a validated food-frequency questionnaire [20]. Adherence to the Mediterranean type of diet was assessed using a dietary score, which has been validated elsewhere [28].
Regarding the rest of the investigated parameters, current smokers were defined as those who smoked at least one cigarette per day, former smokers as those who had stopped smoking for at least one year and the rest of the participants were defined as non-current smokers. To evaluate physical activity, we estimated weekly energy expenditure in sports-related physical activity in terms of frequency (in times per week), duration (in minutes per time) and intensity (in expended calories) [29]. Participants who did not report any physical activities were defined as sedentary.
Height and weight were recorded and body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters). According to standard guidelines, obesity was defined as body mass index >29.9 kg/m2.
Arterial blood pressure was measured in all participants using standard procedures [22], and people whose average blood pressure levels were greater or equal to 140/90 mmHg or who were on anti-hypertensive medication were classified as hypertensive.
Blood samples were collected from the antecubital vein between 8 and 10 a.m., in a sitting position after 12 hours of fasting. Biochemical characteristics (blood glucose levels, serum insulin concentrations, C-reactive protein, serum amyloid A, interleukin-6, homocysteine, tumor necrosis factor-alpha and blood lipids) were measured in our institution's laboratory following established, procedures, as previously described [14, 22, 29]. Diabetes mellitus (type 2) was defined according to the American Diabetes Association diagnostic criteria. Blood glucose levels greater than 125 mg/dl classified participants as having diabetes, while glucose levels between 100 to 125 mg/dl identified impaired fasting glucose (IFG) [21]. Insulin resistance was assessed by the approach involving calculation of a homeostasis model assessment (HOMA) (glucose in mmol/l × insulin in μU/ml / 22.5) [23]. As the ability of the beta-cell to respond to insulin resistance with an increase in hormone secretion is disrupted in type 2 diabetes, especially when insulin-treated, insulin levels were not measured in the samples taken from the diabetic patients on insulin treatment. Moreover, HOMA was not calculated for people with diabetes. A clinically confirmed family history of diabetes was also recorded in all participants. Patients with type 1 were excluded from the analysis because of the small sample size (i.e. < 1% of the study's population). Hypercholesterolemia was defined as total serum cholesterol levels greater than 200 mg/dl or the use of lipid lowering agents.
Data analysis
After applying a statistical power calculation we found that the number of study participants was adequate to evaluate two-tailed standardized differences greater than 0.5 in the investigated inflammatory markers between the diabetic groups. In particular, we achieved statistical power >0.80 at <0.05 probability level (p-value).
Continuous variables are presented as mean ± standard deviation, while categorical variables are presented as absolute and relative frequencies. Associations between diabetic categories and other categorical variables were tested using contingency tables and chi-squared test. Associations between study groups and continuous variables were tested by analysis of variance, or the Kruskal-Wallis test for skewed variables, after checking for equality of variances with the Levene test. Partial correlations between normally distributed continuous variables were evaluated by Pearson's partial r coefficient, after adjusting for age, sex and BMI. Correlations between skewed continuous or discrete variables were evaluated by Spearman's rho-coefficient. The relationships between inflammatory marker levels (dependent variable) and diabetes status or glycemic control indices were examined using multiple linear regression analysis, after controlling for potential confounders. Normality tests were applied using the Shapiro-Wilk criterion. C-reactive protein levels were log-trans-formed because of their skewed distributions. The assumptions of linearity for the continuous independent variables and constant variance of the standardized residuals were assessed by plotting the residuals against the fitted values. As we were making multiple comparisons between diabetic groups, we applied the Bonferroni rule to account for the inflation in type I error. All reported p-values are based on two-sided tests and compared to a significance level of 5%. SPSS 13 (SPSS Inc., Chicago, Il, USA) software was used for all the statistical calculations.
Results
The overall prevalence of diabetes was 118 out of 1514 men (7.8%) and 92 out of 1528 women (6.0%), (p for gender differences = 0.05). Prevalence of IFG was 310 out of 1514 men (21%) and 184 out of 1528 women (12%), (p for gender differences = 0.05). Table 1 shows several characteristics of subjects without diabetes, with IFG and with diabetes. It is evident that subjects in the diabetic group were older, included a higher percentage of males, had a greater BMI and were less physically active than non-diabetic subjects. In addition, diabetic subjects were more likely to be hypertensive and hypercholesterolemic. Smoking habits did not differ between diabetic groups. Moreover, mean HOMA levels in normal people were 2.5 ± 0.48 and in IFG were 3.8 ± 0.43 (p < 0.05; Table 1).
Table 1. Demographic, lifestyle, and clinical characteristics of the participants by diabetes status.
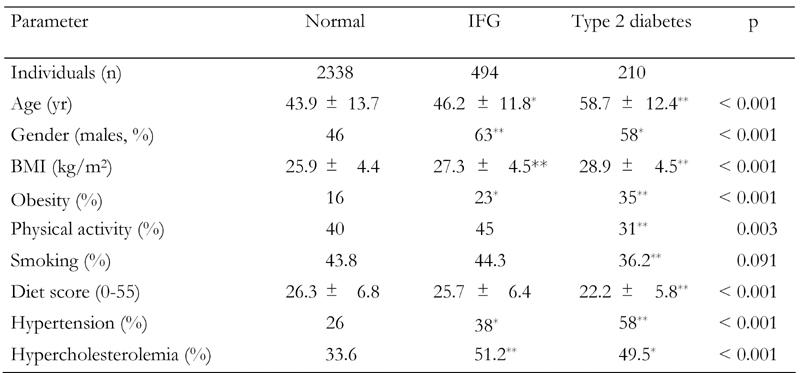
Data are mean ± SD. p from one-way ANOVA. IFG: impaired fasting glucose. * p < 0.05 and ** p < 0.01 for the comparisons between groups in the study (Bonferroni corrected).
Table 2 presents the partial correlation coefficients between blood glucose, insulin, HOMA and inflammatory marker levels. When we stratified the analysis by gender, no between-gender differences were observed in the magnitude of the correlations of glycemic control indices and inflammatory markers. Thus, the analysis was performed after taking into account participants' age, sex and BMI. Blood glucose was positively correlated with almost all inflammatory markers, with the exception of homocysteine. Insulin and HOMA were positively correlated with C-reactive protein, interleukin-6 and tumor necrosis factor-alpha, but not with serum amyloid A. Furthermore, when we stratified the female sub-sample into post-menopausal and others, no differences were observed in the magnitude of the correlations of glycemic control indices and inflammatory markers (Table 2). These data are not given in the text.
Table 2. Partial correlation coefficients between glycemic control indices and inflammatory levels, after adjusting for age, sex and BMI.
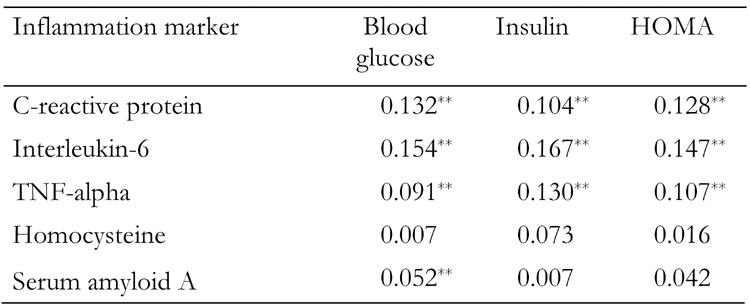
Data are mean ± SD. TNF: tumor necrosis factor. HOMA: homeostasis model assessment. * p < 0.05; ** p < 0.01.
Table 3 presents the comparisons of inflammation markers between the diabetic status groups. As we can see, diabetic subjects had 57% higher mean levels of C-reactive protein (p < 0.001), 22% higher mean levels of interleukin-6 (p < 0.001) and 60% higher levels of tumor necrosis factor-alpha (p < 0.001) compared to non-diabetic subjects. Homocysteine and serum amyloid A levels did not show significant differences between groups (Table 3).
Table 3. Inflammatory markers and diabetes status in the ATTICA study participants.
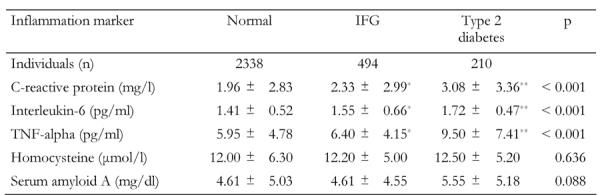
Data are mean ± SD. p from one-way ANOVA. IFG: impaired fasting glucose. TNF: tumor necrosis factor. * p < 0.05 and ** p < 0.01 for the comparisons between groups in the study (Bonferroni corrected).
Although a significant association was previously reported between inflammatory marker levels and glycemic control indices, these relationships may be confounded by several characteristics, especially dietary habits and physical activity level. Thus, we applied multiple regression analysis and, after controlling for several socio-demographic, lifestyle, biochemical and clinical characteristics (i.e. age, sex, BMI, physical activity status, smoking habits, HDL-cholesterol, presence of hypercholesterolemia and hypertension, as well as dietary habits through the diet score), we found positive associations between blood glucose levels and C-reactive protein (p < 0.001) and interleukin-6 (p = 0.002), as well as between insulin levels and C-reactive protein (p = 0.029) and interleukin-6 (p = 0.016). No other significant associations between blood glucose, insulin and inflammatory markers were observed (Table 4).
Table 4. Multiple regression analysis on the association between inflammatory marker levels and glycemic control indices.
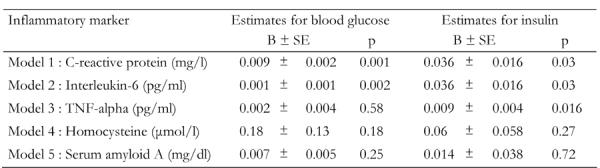
Data are B-coefficient ± SE. Inflammatory markers are dependent, glycemic control indices are independent variables. Values are corrected for various confounders such as age, gender, body mass index, physical activity status, smoking habits, HDL cholesterol, presence of hypercholesterolemia and hypertension, as well as dietary habits through the diet score. TNF: tumor necrosis factor.
Discussion
In this study, we found an association between low-grade inflammation markers and glycemic control indices irrespective of demographic, clinical and lifestyle characteristics, including dietary factors. Specifically, we compared the levels of several inflammatory markers, such as C-reactive protein, interleukin-6, tumor necrosis factor-alpha, homocysteine, and serum amyloid A, between subjects with and without type 2 diabetes mellitus. We found that diabetic subjects had higher C-reactive protein, interleukin-6, and tumor necrosis factor-alpha levels than non-diabetic subjects, whilst homocysteine and serum amyloid A levels did not differ significantly between diabetes groups. In addition, multi-adjusted analysis revealed positive associations of blood glucose and insulin levels with C-reactive protein and interleukin-6 levels, after adjusting for several lifestyle and clinical characteristics.
Our results are consistent with the hypothesis that low-grade inflammation is closely involved in the pathogenesis of type 2 diabetes. We have previously shown that insulin resistance, which predisposes to type 2 diabetes is inversely associated with physical activity status independently of the presence or absence of obesity and that subjects with sedentary lifestyles have higher blood glucose and insulin levels than active subjects from the same BMI group [29]. As physical activity has been negatively associated with the inflammatory process [15-17], we tested its potential confounding role in the relationship between inflammation and glycemic control indices. Our analysis revealed that the positive associations of blood glucose and insulin levels wjth C-reactive protein and interleukin-6 levels were independent of the physical activity status of the participants.
Smoking has been positively associated with several inflammatory markers [26, 27] and could also be considered to be an effect modifier in the association between low-grade inflammation and blood glucose and insulin levels. After controlling for smoking habits, the above-mentioned association did not change.
Dietary habits have long been associated with the management and/or prevention of insulin resistance and diabetes [34]. Previous reports from the ATTICA study have shown that a higher consumption of red meat may aggravate insulin resistance in non-diabetic subjects [30]. To overcome the limitations of the single nutrient approach (i.e. interactions and inter-correlation among nutrients), some investigators have proposed the study of overall dietary patterns [35, 36]. A dietary pattern rich in cereals, fish, legumes, vegetables and fruits has been strongly associated with reduced levels of clinical and biological markers linked to the metabolic syndrome, whereas meat, potato and alcohol intake has shown opposite results [31]. Insulin resistance is a major constituent of the metabolic syndrome [37]. Recently, we have also revealed an inverse association between adherence to the Mediterranean dietary pattern and indices of glucose homeostasis (i.e. glucose, insulin and HOMA levels) in normoglycemic people [32]. Many dietary factors have been reported to activate or inhibit the innate immune system [11-13, 24]. Furthermore, greater adherence to the Mediterranean diet has been independently associated with a reduction in various inflammatory and coagulation markers [14], so it could be argued that diet may also have a confounding role in the association between indices of inflammation and glycemic control. Therefore, we repeated the data analysis by also controlling for participants' dietary habits using the Mediterranean Diet Score. The multivariable analysis confirmed the positive association between C-reactive protein, interleukin-6 and the above-mentioned glycemic indices.
High-density lipoproteins (HDLs) are a class of lipoproteins that promote the efflux of excess cholesterol from cells and return it to the liver for secretion into the bile. Recent studies consider HDLs to be part of the innate immune system, functioning to inhibit inflammation in the absence of an acute phase response [38]. In a previous work from the ATTICA Study, we found an inverse association of C-reactive protein and homocysteine levels with HDL-cholesterol levels, suggesting that increased levels of HDL-cholesterol may lead to decreased low-grade inflammation in healthy individuals [33]. In the present study, diabetic subjects had significantly lower HDL-cholesterol levels than non-diabetic participants. We applied multiple regression analysis by also controlling for HDL-cholesterol and revealed that the positive associations of blood glucose and insulin levels with C-reactive protein and interleukin-6 levels remained unaltered. The independent association found between glucose and insulin levels and the inflammatory markers may well underline the clinical importance of directly targeting inflammation for the treatment and/or prevention of type 2 diabetes.
Limitations
The major limitation of our study is that because of its cross-sectional character, causal relations cannot be established. It can only generate the hypothesis that there is an association between inflammatory markers levels and different glycemic control parameters in our sample. Another limitation is that the biological and nutritional evaluation was performed once, so the accuracy of the data may be prone to measurement error.
Conclusion
In conclusion, our study supports a positive association between low-grade inflammation and diabetes in a population-based sample of men and women without any evidence of cardiovascular disease, which is independent of demographic, clinical and lifestyle characteristics, including physical activity and dietary factors. Further studies are needed to establish a causal pathobiological relationship between glycemic control indices and the inflammation process.
Acknowledgments
The ATTICA study is supported by research grants from the Hellenic Society of Cardiology (grant - 1, HCS2002) and the Hellenic Society of Atherosclerosis. The authors would like to thank the field investigators of the ATTICA study: Natassa Katinioti (physical examination), Akis Zeimbekis (physical examination), Spiros Vellas (physical examination), Efi Tsetsekou (physical/psychologi-cal evaluation), Dina Massoura (physical examination), Labros Papadimitriou (physical examination), as well as the technical team: Marina Toutouza (principal investigator in biochemical analysis), Carmen Vassiliadou (genetic analysis), Manolis Kambaxis (nutritional evaluation), Konstadina Palliou (nutritional evaluation), Constadina Tselika (biochemical evaluation), Sia Poulopoulou (biochemical evaluation) and Maria Toutouza (database management).
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108(24):2993–2999. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- 3.Blake GJ, Ridker PM. Novel Clinical Markers of Vascular Wall Inflammation. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 4.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 6.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharat B, Bairey Merz CN, Sopko G et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109(6):726–732. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 7.Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. C-Reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol. 2003;23(4):650–655. doi: 10.1161/01.ATV.0000065636.15310.9C. [DOI] [PubMed] [Google Scholar]
- 8.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 10.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002;23:831–834. doi: 10.1053/euhj.2001.3052. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ 3rd, Li W, Pagoto SL, Hafner AR, Ockene IS. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83(4):760–766. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonneman G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumour necrosis factor by mononuclear cells. N Engl J Med. 1989;320(5):265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 13.Ganji V, Kafai MR. Third National Health and Nutrition Examination Survey. Demographic, health, lifestyle, and blood vitamin determinants of serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2003;77:826–833. doi: 10.1093/ajcn/77.4.826. [DOI] [PubMed] [Google Scholar]
- 14.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004;44(1):152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 15.Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C. The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study. Prev Med. 2005;40(4):432–437. doi: 10.1016/j.ypmed.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C. Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia. 2006;49(9):2078–2085. doi: 10.1007/s00125-006-0320-6. [DOI] [PubMed] [Google Scholar]
- 17.Borodulin K, Laatikainen T, Salomaa V, Jousilahti P. Associations of leisure time physical activity, self-rated physical fitness, and estimated aerobic fitness with serum C-reactive protein among 3,803 adults. Atherosclerosis. 2006;185(2):381–387. doi: 10.1016/j.atherosclerosis.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Verdaet D, Dendae P, De Bacquer D, Delanghe J, Block P, De Backer G. Association between leisure time physical activity and markers of chronic inflammation related to coronary heart disease. Atherosclerosis. 2004;176(2):303–310. doi: 10.1016/j.atherosclerosis.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Trichopoulou A. From research to education: the Greek experience. Nutrition. 2000;16:528–531. doi: 10.1016/s0899-9007(00)00346-4. [DOI] [PubMed] [Google Scholar]
- 20.Katsouyanni K, Rimm EB, Gnardellis D, Trichopoulos D, Polychronopoulos E, Trichopoulou A. Reproducibility and relative validity of an extensive semi-quantitative food frequency questionnaire using dietary records and biochemical markers among Greek schoolteachers. Int J Epidemiol. 1997;26:S118–S127. doi: 10.1093/ije/26.suppl_1.s118. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 22.Pitsavos C, Panagiotakos DB, Chrysohoou C, Stefanadis C. Epidemiology of cardiovascular risk factors in Greece: aims, design and baseline characteristics of the ATTICA study. BMC Public Health. 2003;3:32. doi: 10.1186/1471-2458-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T, Itoh H, Doi K, Fukunaya Y, Hosada K, Shintani M, Yamashita J, Chun TH, Inoue M, Masatsugu K et al. Down regulation of peroxisome proliferator-activated receptor γ expression by inflammatory cytokines and its reversal by thiazolidinediones. Diabetologia. 1999;42(6):702–710. doi: 10.1007/s001250051218. [DOI] [PubMed] [Google Scholar]
- 26.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26(17):1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 27.Woodward M, Rumley A, Tunstall-Pedoe H, Lowe GD. Associations of blood rheology and interleukin-6 with cardiovascular risk factors and prevalent cardiovascular disease. Br J Haematol. 1999;104(2):246–257. doi: 10.1046/j.1365-2141.1999.01158.x. [DOI] [PubMed] [Google Scholar]
- 28.Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2006;16(8):559–568. doi: 10.1016/j.numecd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Kavouras SA, Panagiotakos DB, Pitsavos C, Chrysohoou C, Anastasiou CA, Lentzas Y. Physical activity, obesity status, and glycemic control: The ATTICA study. Med Sci Sports Exerc. 2007;39(4):606–611. doi: 10.1249/mss.0b013e31803084eb. [DOI] [PubMed] [Google Scholar]
- 30.Panagiotakos DB, Tzima N, Pitsavos C, Chrysohoou C, Papakonstantinou E, Zampelas A, Stefanadis C. The relationship between dietary habits, blood glucose and insulin levels among people without cardiovascular disease and type 2 diabetes; The ATTICA Study. Rev Diabet Stud. 2005;2(4):208–215. doi: 10.1900/RDS.2005.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panagiotakos DB, Pitsavos C, Skoumas Y, Stefanadis C. The association between food patterns and the metabolic syndrome using principal components analysis: The ATTICA Study. J Am Diet Assoc. 2007;107(6):979–987. doi: 10.1016/j.jada.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Panagiotakos DB, Tzima N, Pitsavos C, Chrysohoou C, Zampelas A, Toussoulis D, Stefanadis C. The association between adherence to the Mediterranean diet and fasting indices of glucose homoeostasis: the ATTICA Study. J Am Coll Nutr. 2007;26(1):32–38. doi: 10.1080/07315724.2007.10719583. [DOI] [PubMed] [Google Scholar]
- 33.Chrysohoou C, Pitsavos C, Skoumas J, Masoura C, Katinioti A, Panagiotakos D, Stefanadis C. The emerging anti-inflammatory role of HDL-cholesterol, illustrated in cardiovascular disease free population; the ATTICA study. Int J Cardiol. 2006 doi: 10.1016/j.ijcard.2006.11.010. In press. [DOI] [PubMed] [Google Scholar]
- 34.Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehto J, Lindstroem J, Louheranta A. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 2004;7(1A):147–165. doi: 10.1079/phn2003586. [DOI] [PubMed] [Google Scholar]
- 35.Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr. 2001;73(1):61–67. doi: 10.1093/ajcn/73.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs DR Jr, Steffen LM. Nutrients, foods and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78(3S):5085–5135. doi: 10.1093/ajcn/78.3.508S. [DOI] [PubMed] [Google Scholar]
- 37.Jarrett RJ. The metabolic syndrome. Lancet. 2005;366(9501):1922. doi: 10.1016/S0140-6736(05)67779-3. [DOI] [PubMed] [Google Scholar]
- 38.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Hama S, Hough G, Bachini E, Grijalva VR, Wagner AC et al. The double jeopardy of HDL. Ann Med. 2005;37(3):173–178. doi: 10.1080/07853890510007322. [DOI] [PubMed] [Google Scholar]


