Abstract
Significant differences in colon cancer incidence worldwide have led to the hypothesis that this variation can be explained largely by environmental, notably dietary influences. Although a positive correlation between dietary fat intake and incidence is suggested from some human epidemiological and rodent carcinogenesis studies, a direct association remains contentious. Using a spontaneous mouse tumor model of multiple intestinal neoplasia, we demonstrate that there is a generalized increase in tumor counts, in both the large and small bowel with higher dietary fat [standard (3%) fat versus high (15%) fat diet (mean ± SD) 1.59 ± 1.46 vs. 3.85 ± 2.37 P < 0.001 and 21.36 ± 7.4 vs. 31.3 ± 9.7, respectively, P < 0.001]. Increasing dietary fat also increases polyp size in the small bowel. These changes appear independent of total calorific intake as assessed by body weights. Halving the crude fiber intake together with an increase in dietary fat from 3% to 10% did not have as marked an effect on tumor counts as an increase of fat alone to 15%, which also decreased survival (P < 0.05). These results demonstrate that increasing dietary fat intake from weaning can have a significant adverse effect on polyp numbers in mice genetically predisposed to intestinal tumor development. A further understanding of the biology of this interaction may provide novel strategies aimed at both colonic polyp prevention and treatment.
In the developed world, colorectal cancer (CRC) vies with prostate cancer as the commonest nonsmoking related cause of cancer deaths. A remarkable 20-fold difference in incidence worldwide, has led to the attractive hypothesis that this variation can be explained largely by environmental influences (1). Studies of normally lower risk populations show that they can take on the higher host risk even within a migrating generation. Claims that up to two-thirds of such incidence variations are accounted for by diet, notably changes in the fat and fiber content, have been difficult to substantiate. The major difficulty in unravelling dietary epidemiological and analytical data is whether the observed trends can be ascribed to a specific class of, or a single constituent, nutrient. Indeed, interventional prospective studies have often failed to reveal a reduction in risk (2).
Despite strong epidemiological evidence of an association between dietary fat intake and colorectal cancer incidence, a causal relationship continues to be questioned. Since fat intake is predominantly sourced from red meat, there maybe other associated ingestants that are responsible for the apparent increased risk (3). Dietary intake of other constituents such as fiber and micronutrients may also mitigate against this risk. There are obvious major limitations in prospectively studying interventional environmental influences in human colorectal cancer. These include the accessibility and availability of sufficient numbers of patients and study material for statistical validation, the relatively long clinical history of disease in some patients, the ethical barriers to initiating double-blind controlled interventions, and the subsequent intensive monitoring of their effects. Study populations, being genetically heterogeneous, may further confound analyses by the complexities of different environmental exposures prior to, and often during trials.
Familial adenomatous polyposis (FAP) is an autosomal dominant disorder in humans characterized by the development of numerous colorectal polyps with invariable development to colonic carcinoma by middle age. Germ-line mutations of the APC (adenomatous polyposis coli) gene are responsible for FAP (4–6). Although it accounts for less than 0.5% of all colorectal malignancies overall, it remains a good investigative model for sporadic disease, as mutations in APC are often also present as the earliest genetic alteration in most sporadic colon carcinomas (7).
Carcinogen-induced intestinal tumors in rodents, using dimethylhydrazine (DMH) or azoxymethane, have been the favored animal model of CRC. Studies with dietary fat augmentation in such models have generally, but not invariably, shown adverse effects (8). The variability of scheduling and dosage of administration of DMH, the rodent strain used, and type and timing of fat augmentation, make interpretation difficult. Additionally, although DMH-induced invasive tumors in rodents have been shown to have Ras mutations, they rarely have Apc mutations or P53 allelic loss, questioning the relevance of this carcinogenic model to human CRC (9). In support of this, DMH-induced CRC susceptibility loci mapped in recombinant congenic mice do not appear to involve Apc or P53 (10).
The multiple intestinal neoplasia (MIN) mouse has a fully penetrant dominant phenotype (11), which cosegregates with a germ-line nonsense mutation (codon 850: leu (TTG) > stop (TAG)) in the murine homologue of the APC gene (Apc) (12). This mutation is analogous to those found in human FAP kindreds and in sporadic colorectal cancers. APC murine and human coding sequences are 86% and 90% identical at the nucleotide and amino acid levels, respectively, with conservation of important motifs. Homozygote MIN mice are embryonic lethals (13), but heterozygotes on a C57BL/6J background develop numerous predominantly small intestinal adenomatous polyps within the first 3 months of life. There is also an increased incidence of non-mouse mammary tumor virus-dependant mammary tumors, which remains unexplained as neither FAP nor APC mutations are obviously associated with breast tumors in humans (14). The MIN mouse thus provides the first spontaneous tumor model for examining approaches to the prevention and treatment of intestinal polyps.
We present here the effects of increasing dietary fat on intestinal polyp formation and survival in this spontaneous intestinal tumor mouse model.
MATERIALS AND METHODS
Establishment of the Imperial Cancer Research Fund (ICRF) MIN C57/BL 6J Colony.
MIN (C57BL/6JApcMin/+Apc) heterozygote mice were originally obtained in 1992 (gift from A. Moser, McArdle Labs, University of Wisconsin, Madison). Male mice were initially back-crossed to female C57BL/6J (ICRF Biological Resources, Clare Hall, South Mimms, Hertfordshire, United Kingdom), and the resultant embryos were transferred by aseptic hysterectomy to foster mothers in specified pathogen free isolators. All breeding was subsequently performed in specified pathogen free units by brother (C57BL/6J-ApcMin/+Apc)-sister (C57BL/6J)-mating.
Genotyping was carried out by a PCR-based reaction using three primers including an internal control for normal mouse DNA as described (15). DNA was extracted from ear or tail snips at 21 days of age. Briefly, ear snips were put in 20 ml of freshly prepared lysis buffer [12.5 μl 1 M Tris (pH 8)/1 μl 5 M NaCl/2.5 μl 10% SDS/25 μl 10 mg/ml proteinase K/250 μl autoclaved double-distilled H2O] and mixed and heated in a 55°C bath for 1 hr, mixed again, and heated for a additional 2 hr, and transferred to ice. Eighty microliters of ddH2O was added and the digest boiled for 15 min to inactivate the proteinase K. Next, 2.5 μl of this mixture was used for a 25-μl PCR. Alternatively, 1 cm of fragmented mouse tail (obtained under anaesthesia) was placed in tail lysis buffer (700 μl TENs/35 μl 20% SDS/35 μl of 10 mg/ml proteinase K) and incubated overnight at 56°C. DNA was obtained by standard phenol/chloroform extraction with isopropanol precipitation and stored in 250 μl TE. One-half microliter of this mixture was used for a 25-μl PCR.
Two hundred and twenty-nine (C57BL/6J-ApcMin/+Apc) (MIN) and 23 (C57BL/6J-+Apc/+Apc) (wild-type) control mice were randomly stratified prospectively (from over 50 matings) into 3 dietary groups as follows:
(i) A “Standard” diet with approximately 3% total fat content (ICRF Rodent diet GR3EK.R20, Special Dietary Services, Witham, Essex, United Kingdom). The crude fiber content of this diet (as assessed by the acid/alkali extraction technique) is 4.1% and the carbohydrate and crude protein contents 58% and 19.8%, respectively.
(ii) A “Fat” diet with a minimum 10% total fat content [Teklad mouse breeder diet(W) 8626; Madison, WI] obtained from the same source as that used in the first published data on MIN (11). Constituents other than the fat content of this diet also varied from diets (i) and (iii) in that the crude fiber content is halved to 2%, although the crude protein is similar at 20%.
(iii) A “High Fat” diet with approximately 15% total fat content. This was specifically reconstituted by SDS from 85% by weight of the standard ICRF Rodent GR3EK.R20 diet (i.e., as diet i above), with an additional 15% corn oil by weight. The four main fatty acid constituents of the corn oil used are: 42.9% linoleic, 23.4% oleic, 9.8% palmitic, and 2% steric.
The standard (i) and high fat (iii) diets are therefore similar apart from their total fat contents, whereas diet (ii) Fat differs in fat and has a lower fiber composition. The range of diets was selected in part to help account for the substantially lower polyp numbers and increased survival that we had originally observed in our ICRF MIN mice, as compared with the originally published data from which our MIN mice were sourced (11).
Diets were randomly allocated and initiated ad libitum from weaning at approximately 21 days of age, after their transfer to dedicated isolators housed in a separate “open” unit (i.e., under normal nonspecified pathogen free animal laboratory conditions). No attempted adjustment for calorific dietary values was made, because mouse end-calorific intake and utilization is not easily controllable or assessable, and is further compounded by the potential differences in palatability between the three diets. There have also been reports of abnormal dietary handling, including obesity resistance in the C57BL laboratory mouse for which the basis is unclear (16). The mice were however weighed in their stratified groups on a weekly basis, from weaning until sacrifice. C57BL/6J-+Apc/+Apc mice of each sex were also randomly allocated as negative controls within each dietary group to exclude possible unexpected adverse dietary effects and also as positive controls for weight comparisons. This was to ensure that our observations were not confounded by detrimental effects independent of the MIN phenotype or conversely, abnormal dietary handling that might be MIN dependant.
The need for sacrifice was determined by independent technicians who assessed the mice daily. MIN mortality is usually due to severe progressive anemia, rectal prolapse, or intestinal obstruction. Progression of a mammary tumor to beyond 1 cm in diameter also necessitates sacrifice. When possible, post-mortems, counting, and measurements were carried out single-blind by only one person (H.S.W.), otherwise tumor measurements were disregarded. At post mortems the intestine was isolated, dissected longitudinally, washed three times in PBS, and spread onto 3MM paper. The small bowel was divided into three approximately equal sections [proximal (SB1), middle (SB2), and distal (SB3)]. An assessment of the visible number, size [mean of two largest diameters with digital callipers (Micron Sales, London)], and distribution of intestinal tumors was then made under a ×10 dissecting microscope. The accuracy of tumor size resolution by this approach necessitated counts per 1-mm interval. Sections were then fixed into 9% formal saline and embedded in paraffin for histological evaluation. All procedures are approved by the ICRF animal ethics committee.
Statistical Analyses.
Data were entered into Microsoft excel 5.0 and subsequently analyzed using stata 4.0 (Timberlake, Kent, United Kingdom). Comparisons of survival by diet used Kaplan–Meier and log-rank analyses and were cause-specific (not including mice sacrificed for mammary tumors). Tumor numbers were compared with Wilcoxon’s rank-sum test. For tumor size, the proportion of tumors larger than 3 mm in diameter was compared by Pearson’s χ2 test.
RESULTS
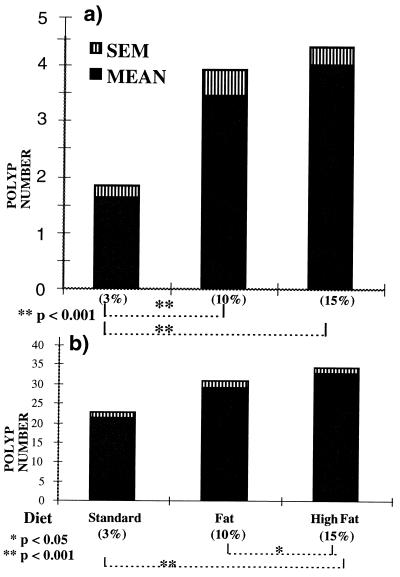
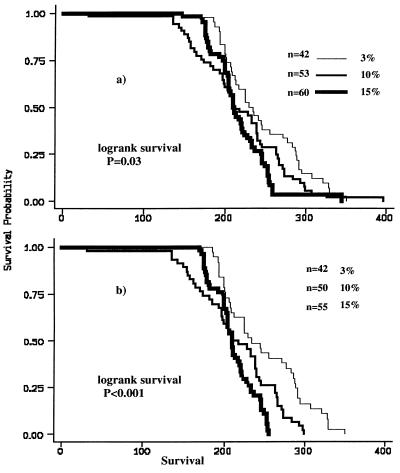
Our data show that increasing the dietary fat content from weaning, has significant adverse effects on the number of large and small bowel polyps, and on the polyp-specific survival in the MIN mouse (Figs. 1 a and b and 2a). These effects are all sex independent. There is a consistent trend in the comparison of the Standard 3% fat diet with increasing dietary fat for all three of these variables, but the differences in survival only attain statistical significance between the lowest (3%) fat and the High (15%) fat diets. However, Fig. 2b shows the effect on the survival curves when five (from 15%) and three (from 10%) mice were excluded from the survival curves (“outliers” on residual analysis). The wild-type control C57BL/ 6J-+Apc/+Apc littermates had a mean survival beyond 500 days, irrespective of their different diets. This is significantly greater than the average survival for any of the C57BL/6J-Min/+Apc dietary groups (p < 0.001, log-rank comparisons; Table 1). Most negative controls were specifically sacrificed randomly after 500 days to ensure that they had not developed any intestinal tumors, which was confirmed histologically. This suggests that the observed decrease in MIN survival with increasing dietary fat, was directly due to the increased assessable tumor burden.
Figure 1.
(a) Large and (b) small bowel polyp counts in MIN (C57BL/6J-Min/+Apc) mice on three different diets varying in their fat composition. Comparisons by Wilcoxon’s rank-sum tests.
Figure 2.
(a) Kaplan–Meier survival curves of MIN (C57BL/ 6J-Min/+Apc) on three different diets varying in their fat composition. (b) The effect on the curves when eight animals are excluded (“outliers” by residual analysis). Note that five of these mice are from one parent.
Table 1.
Summary table of collective data presented in text and figures
| Dietary Fat
|
||||
|---|---|---|---|---|
| Assessable n | 3% | 10% | 15% | |
| Overall survival, days | 181 | 226.9 | 208.1 | 209 |
| Excluding mammary | 155 | 244.1 | 218.6 | 217.4 |
| Wild type | 23 | >500 | >500 | >500 |
| % mammary tumors | 229 | 41.3 | 20 | 26.5 |
| Small bowel polyp, no. | 145 | 21.3 | 27 | 31.8 |
| Large bowel polyp, no. | 145 | 1.83 | 3.78 | 4.12 |
| Polyps > 3 mm SB, % | 89 | 25.7 | 40.7 | 31.1 |
| Polyps > 3 mm LB, % | 89 | 38.5 | 32 | 32.8 |
The differential distribution and diameter of polyps across three equal segments of small bowel (SB1, -2, -3, respectively) was assessed in 30, 26, and 33 mice from the 3%, 10%, and 15% fat groups. This showed no specific segment within the small bowel that was preferentially affected in terms of polyp counts by the changes in dietary fat (data not shown). There was a significant increase in the proportion of tumors larger than 3 mm in the whole of the small bowel when 3% dietary fat was compared with either 10% or 15% fat [25.7% vs. 40.7%, P < 0.001 and 25.7% vs. 31.1%, P = 0.013, respectively (Table 1)]. The 10% fat diet had a significant tumor enlarging effect within all three segments of the small bowel, whereas the 15% diet effect was only significant within one segment (SB2). The magnitude of increase was also proportionately larger for the 10% as compared with 15% diet. None of the diets enlarged tumors in the colorectum.
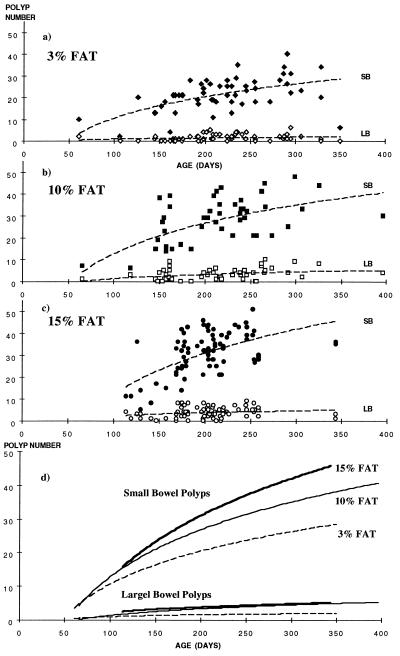
In comparing tumor occurrence by age, there appears to be a proportional, almost linear, increase within the three different dietary groups. This effect is clearer for the small than the large bowel (Figs. 3 a–c). Fig. 3d superimposes individual logarithmic regression lines and suggests that the rate of increase of polyp numbers with age is higher with increasing dietary fat, but the differences between the groups are only likely to be apparent after approximately 100 days of age. The differences also appear to increase until sacrifice, suggesting either that new tumors continued to develop throughout life or that a higher proportion of tumors attain our detectable size threshold. These data suggest that an age-adjusted correction for comparing polyp counts would have the effect of accentuating the differences further. Thus, mice on lower fat diets would have an even lower age-adjusted polyp count as they also survived longer than those in the higher fat groups, and vice versa.
Figure 3.
(a–c) Small (solid symbols) and large (open symbols) bowel polyp counts in MIN (C57BL/6J-Min/+Apc) mice vs. age on the three different diets with best-fit logistic regression lines. (d) Logarithmic regression comparisons between the three different diets from the individual data in a–c (i.e., three graphs superimposed).
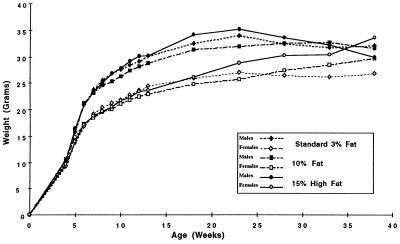
As the diets were not isocalorific, the mean weekly weights of a sample of each group of mice are shown in Fig. 4. The overall mean weights between the 3%, 10%, and 15% dietary fat groups were not significantly different throughout their lifespans or as assessed by maximum attained weights (data not shown). A divergence trend is apparent after about 15 weeks of age but is confounded by a gradual drop in weights from around 24 weeks due to MIN-dependant morbidity and larger variation, since the number of assessable mice progressively decreased. The weight patterns of the equivalent groups of C57BL/6J-+Apc/+Apc controls were again similar up to about 20 weeks (data not shown), demonstrating that Apc is not likely to be involved directly in intestinal absorption or metabolism.
Figure 4.
Mean weights of MIN (C57BL/6J-Min/+Apc) mice stratified by sex on the three different diets varying in their fat composition. The weights are plotted at five-weekly intervals from weaning (from ≈3 weeks of age).
Histology.
Apart from size, no obvious differences were found between the dietary groups, either in the macro- or microscopic appearance on hematoxylin/eosin sections of small or large bowel polyps. Local invasion into the lamina propria is rare in MIN but appears commoner in the larger polyps, and was not observed in tumors smaller than 3 mm in diameter. No obvious increase in invasive tumors was observed with higher dietary fat. No metastases were seen microscopically in mesenteric lymph nodes, liver, or lung in a random sample of the mice.
Breast Tumors and Diet.
Twenty-seven mammary tumors occurred from 93 females overall (29%). There was no increase in breast tumors seen on the 15% fat (26.5%) as compared with the 3% fat diet (41.3%, 9 tumors per 34 females vs. 12 tumors per 29 females). Although there were only 6 tumors per 30 females (20%) in the 10% fat diet group, this incidence was not significantly different to the other two groups by Pearson’s χ2. The mean age of mammary tumor-specific sacrifice was significantly less than the overall group survival (see Table 1), but was again diet independent. Sacrifice due to the unpredictable onset of prolapsed rectal polyps was also diet independent and had no bearing on any of our endpoints (data not shown).
DISCUSSION
The survival of the first 100 C57BL/6J MIN mice at the ICRF was significantly longer, with correspondingly fewer small and large bowel polyps as compared with the originally published data [223 ± 43 days, mean ± SD (ICRF-MIN) vs. 119 ± 31 days] (11) from where our MIN mice were directly sourced. This suggested that there may be strong environmental modifying factors influencing tumor initiation and development in the MIN mouse model. We therefore set out to investigate in a single-blind randomized controlled trial some of the possible reasons for the apparent marked differences by altering the dietary fat content of our MIN mice and also reinstituting the same diet that was used in the original publication.
Our results demonstrate significant deleterious effects of increasing dietary fat on the spontaneous intestinal tumor MIN mouse model when bred on the C57BL/6J background. This concurs with some human epidemiological studies of the effect of dietary fat on the incidence of sporadic colorectal cancer (17). Increasing the dietary fat by the addition of 15% by weight of corn oil resulted in a significant increase in both intestinal adenoma numbers and their size at sacrifice. For polyp numbers the increase was proportionally greater in the large as compared with the small bowel. Increasing dietary fat was also associated with a reduced survival, concordant with tumor burden. However, these deleterious effects of fat did not obviously translate into a higher proportion of tumors being invasive, nor of a more undifferentiated histological phenotype. There were smaller differences between the Standard (3%) ICRF and 10% Fat diet groups, which were not always statistically significant. As the increase in dietary fat was intermediate in this case, it therefore may not have been sufficient to increase tumor burden discernibly. Alternatively, as there are other differences between these two diets, such as the source and types of fatty acids, these may have mitigated against any adverse effects of the increased total dietary fat. Interestingly, the 10% Fat diet (Harlan–Teklad prepared) was also lower in crude fiber (2%) than the two ICRF (SDS prepared) diets (4% fiber), so the observed result of a smaller difference between the standard 3% Fat and 10% Fat diets is perhaps more surprising.
Our results suggest that diet alone cannot account for the relatively large differences observed between our lower tumor counts and the originally published data (11). It is possible that strain differences between colonies are also partially responsible, despite their C57BL/6J label. Indeed, intrastrain variation is suggested by Fig. 2b, because five of the eight outliers were related. On the other hand, we have not observed any significant change in tumor counts over time with our ICRF MIN mice, which were originally from Wisconsin. Specifically, there was no rapid decrease in tumor counts with the early generations as might be expected if strain differences were largely responsible (personal observations). Another possible explanation is that diet is only one side of the equation and that gut microflora, which is known to play a significant role in interacting with dietary constituents, plays a critical role in influencing the tumor micro-environment. We have already observed significant changes in this regard in our own MIN colonies housed in facilities with different stringencies for microbial avoidance.§
C57BL/6J mice are known to be homozygous null for secretory phospholipase A2 (Pla2s), a luminally secreted intestinal enzyme known to have direct lipid-metabolizing activity. Pla2s is within the candidate region for MOM-1 (a modifier of MIN), which can influence about 50% of the genetic variation in eventual tumor number attributable to Min/+Apc (15). Despite the presence of the loss of heterozygosity of human Pla2s (1p35–36) in about a one-third of sporadic CRCs and equivocal evidence of modifying activity in duodenal disease in FAP (18), no Pla2s mutations have yet been identified in sporadic CRC. Enzymes involved in phospholipid and so dietary fat metabolism, which also demonstrate direct anti-bacterial properties, may provide an important link to understanding environmental influences on tumor development. For example, they may contribute to the regulation of luminal paracrine signaling molecules that directly act on gastrointestinal epithelium, so influencing its rate of turnover. In this regard, dietary lipids have been shown to alter the production of the E-series of prostaglandins and influence the metabolic activity of fecal microflora and concentrations of intestinal sterol substrates. Bacterial fermentation of carbohydrates and “Fiber” can lead to the production of short chain fatty acids, such as butyric acid, which can exert strong trophic effects throughout the gastrointestinal tract (19). Thus, an understanding of the control of intestinal cell turnover influenced by interactions between dietary constituents, the intestinal microflora, and genetic factors may help to unravel critical steps in tumor development.
Most animal studies to date have centered on rodent carcinogenesis models. C57BL/6J is a relatively resistant mouse strain to the induction of colonic adenocarcinomas by DMH (20). Conversely, it is a highly sensitive strain to tumor development with Min/+Apc (15, 21). Temple and El-Katib showed that a high fat diet acted as a promotional factor when given after DMH-treatment was stopped, but if given simultaneously with DMH administration only slightly increased the incidence of colon tumors (8). Surprisingly, 3 of 36 C57BL/1 mice fed a diet supplemented only with corn oil and oleic acid developed carcinomas with metastases, normally a very rare occurrence in mice (22). Our high fat supplementation was also based on corn oil, but we did not observe any metastases in our study population. The problem with carcinogen studies, as discussed earlier, is that the relationship between fat intake and experimental animals remains contradictory and controversial (17). Although a prospective study in nurses did show a significant risk for dietary fat after adjustment for total caloric intake (23), a causal role in other human experimental and case control studies has not always been confirmed (24), and indeed has even shown an inverse association (25–27).
There are suggestions that the specific type of dietary fat may influence CRC risk, and it may be that the proportion of saturated fat intake is important (28), but again not all case control studies show this (29). A protective effect of monounsaturated fat and linoleic acid [an n6-polyunsaturated fatty acid (PUFA)] has been found in some studies (25, 30), whereas PUFAs from vegetable, but not fish oils, had adverse effects in others (31). Fish oils (containing eicosapentaneoic acid, an omega-3 PUFA) and olive oil (monounsaturated oleic acid) have also been shown to have an inhibitory effect of tumor development in animals (31), including docosahexaenoic acid in the MIN mouse model (32). All dietary experiments may also be confounded by nonfat food variables, for example, a high fiber intake often reduces the magnitude of the effect of fat intake on CRC incidence in humans (28). In our experiment, increasing the fat over 3-fold (from 3% to 10%) and halving the crude fiber content (from 4% to 2%) had no significant effect on survival, (or small bowel polyp numbers), but did significantly increase the large bowel polyp numbers almost 2-fold. This suggests that dietary influences on tumor development are different in the large vs. the small bowel, which may partially account for the 100-fold reduced frequency of sporadic small bowel tumors in man. Our findings should thus influence the dietary advice given to FAP patients, since both rectal and duodenal recurrences are major causes of morbidity after colectomy.
Our data suggest that tumors can be initiated by increasing dietary fat. Carcinogenesis studies in MIN show that the period most likely to influence tumor development is before the first 3 weeks of life. After 3 weeks, a weaker influence can be observed on polyp initiation (33). Our diets were initiated after weaning and, therefore, this cannot also be the critical period for their influence. However, because our method does not detect tumors <0.5 mm in diameter, the observed dietary effects may alternatively represent a shift in proportion from smaller to larger tumors through adult life. Thus, the data are inconclusive as to the role of dietary fat on tumor initiation vs. promotion.
There is some evidence that an increased calorie intake per se may have deleterious effects on tumor growth and, conversely, that calorie restriction can reduce tumor incidence in laboratory animals (34–36) and prolong survival in P53 null mice (37). In humans, the evidence is less certain (38). Our experiment was not isocalorific, because the exact dietary intake would be both difficult to control and ascertain accurately. Additionally, the physical consistency and possibly also the palatability of the diets was different. Furthermore, dietary intake regulates satiety leading to a physiologic calorie adjustment. Using weight as a marker, MIN mice on the three diets did not have statistically significant differences in weight during their lifespans, which was perhaps initially surprising. However, one of the diets (10% fat) has other major constituent differences. Fig. 3d suggests that polyp number differences between the three diets are already apparent by 100 days. Published data suggest that tumor formation occurs before 60 days (39). The early effects of our diets are therefore clearly independent of end-calorific intake as judged by mean weights. This would suggest that our observations are due directly to the proportion of fat consumed and are not, at least initially, calorie dependant. The proportion of tumors larger than 3 mm was highest in the 10% fat group, so this effect may have been due to differences other than the fat content in this diet.
Cellular based experiments have shown both enhanced proliferative or suppressive effects, depending on the specific lipids investigated. These observations are further confounded by the questionable relevance of in vitro proliferation studies to intestinal carcinogenesis. Spontaneous tumor-forming animal models with genetic defects akin to those identified in human disease may be a better approach to studying the often complex effects of environmental influences, such as diet. Cause-specific mortality can be also be assessed as an endpoint, which is of more relevance in experimental design, when extrapolating to human studies.
In conclusion, increasing dietary fat intake has adverse effects on tumor numbers, size, and survival in the MIN mouse. The mechanism and overall significance of these effects to humans are unclear and are likely to be complex, but may help provide an exploitable future strategy for tumor prevention and treatment. Further experiments to ascertain the mechanism of these effects, particularly the potential interaction with the gut microflora, are underway.
Acknowledgments
We are very grateful to Profs. A. Moser and W. Dove (McArdle Labs, Madison, WI) for providing the original stock of MIN mice to the ICRF. We are also most grateful to the Biological Resources unit (ICRF) and the ICRF Histopathology and Statistics units. H.S.W. was funded both by a Medical Research Council Clinical Training fellowship and the ICRF.
ABBREVIATIONS
- MIN
multiple intestinal neoplasia
- APC
adenomatous polyposis coli
- FAP
familial adenomatous polyposis
- CRC
colorectal cancer
- DMH
dimethylhydrazine
- ICRF
Imperial Cancer Research Fund
Footnotes
American Association of Cancer Research Keystone Meeting, February 1996, 1996, A52, abstr.
References
- 1.Potter J D. Eur J Cancer. 1995;31:1033–1038. doi: 10.1016/0959-8049(95)00125-3. [DOI] [PubMed] [Google Scholar]
- 2.Trock B, Lanza E, Greenwald P. J Natl Cancer Inst. 1990;82:650–661. doi: 10.1093/jnci/82.8.650. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Rimm E B, Stampfer M J, Colditz G A, Ascherio A, Willett W C. Cancer Res. 1994;54:2390–2397. [PubMed] [Google Scholar]
- 4.Bodmer W F, Bailey C J, Bodmer J, Bussey H J, Ellis A, et al. Nature (London) 1987;328:614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- 5.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, et al. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 6.Kinzler K W, Nilbert M C, Su L K, Vogelstein B, Bryan T M, et al. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 7.Cottrell S, Bicknell D, Kaklamanis L, Bodmer W F. Lancet. 1992;340:626–630. doi: 10.1016/0140-6736(92)92169-g. [DOI] [PubMed] [Google Scholar]
- 8.Temple N J, El-Khatiib K S. Cancer Lett. 1987;37:109–114. doi: 10.1016/0304-3835(87)90151-0. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto M, Ohtsu H, Miyaki M, Yonekawa H. Carcinogenesis. 1993;14:1483–1486. doi: 10.1093/carcin/14.7.1483. [DOI] [PubMed] [Google Scholar]
- 10.Moen C J, Groot P C, Hart A A, Snoek M, Demant P. Proc Natl Acad Sci USA. 1996;93:1082–1086. doi: 10.1073/pnas.93.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moser A R, Pitot H C, Dove W F. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 12.Su L K, Kinzler K W, Vogelstein B, Preisinger A C, Moser A R, Luongo C, Gould K A, Dove W F. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 13.Moser A R, Shoemaker A R, Connelly C S, Clipson L, Gould K A, Luongo C, Dove W F, Siggers P H, Gardner R L. Dev Dyn. 1995;203:422–433. doi: 10.1002/aja.1002030405. [DOI] [PubMed] [Google Scholar]
- 14.Moser A R, Luongo C, Gould K A, McNeley M K, Shoemaker A R, Dove W F. Eur J Cancer. 1995;31:1061–1064. doi: 10.1016/0959-8049(95)00181-h. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich W F, Lander E S, Smith J S, Moser A R, Gould K A, Luongo C, Borenstein N, Dove W. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 16.Fenton P F, Dowling M T. J Nutr. 1953;49:319–331. doi: 10.1093/jn/49.2.319. [DOI] [PubMed] [Google Scholar]
- 17.Willett W C, Stampfer M J, Colditz G A, Rosner B A, Speizer F E. N Engl J Med. 1990;323:1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 18.Tomlinson I P, Neale K, Talbot I C, Spigelman A D, Williams C B, Phillips R K, Bodmer W F. J Med Genet. 1996;33:268–273. doi: 10.1136/jmg.33.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakata T, Yajima T. Q J Exp Physiol. 1984;69:639–648. doi: 10.1113/expphysiol.1984.sp002850. [DOI] [PubMed] [Google Scholar]
- 20.Evans J T, Hauschka T S, Mittelman A. J Natl Cancer Inst. 1974;52:999–1000. doi: 10.1093/jnci/52.3.999. [DOI] [PubMed] [Google Scholar]
- 21.Moser A R, Dove W F, Roth K A, Gordon J I. J Cell Biol. 1992;116:1517–1526. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Khatiib K S, Cora E M. J Natl Cancer Inst. 1981;66:297–301. [PubMed] [Google Scholar]
- 23.Willett W. Nature (London) 1989;338:389–394. doi: 10.1038/338389a0. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong B, Doll R. Int J Cancer. 1975;15:617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 25.Tuyns A J, Kaaks R, Haelterman M. Nutr. Cancer. 1988;11:189–204. doi: 10.1080/01635588809513986. [DOI] [PubMed] [Google Scholar]
- 26.McKeown E G, Bright S E. Nutr Cancer. 1985;7:251–253. [Google Scholar]
- 27.Boyle P, Zaridze D G. Ecol Dis. 1983;2:241–248. [PubMed] [Google Scholar]
- 28.Hursting S D, Thornquist M, Henderson M M. Prev Med. 1990;19:242–253. doi: 10.1016/0091-7435(90)90025-f. [DOI] [PubMed] [Google Scholar]
- 29.Potter J D, McMichael A J. J Natl Cancer Inst. 1986;76:557–569. doi: 10.1093/jnci/76.4.557. [DOI] [PubMed] [Google Scholar]
- 30.Macquart M G, Riboli E, Cornee J, Charnay B, Berthezene P, Day N. Int J Cancer. 1986;38:183–191. doi: 10.1002/ijc.2910380207. [DOI] [PubMed] [Google Scholar]
- 31.Carroll K K. Am J Clin Nutr. 1991;53:1064s–1067s. doi: 10.1093/ajcn/53.4.1064S. [DOI] [PubMed] [Google Scholar]
- 32.Oshima M, Takahashi M, Oshima H, Tsutsumi M, Yazawa K, Sugimura T, Nishimura S, Wakabayashi K, Taketo M M. Carcinogenesis. 1995;16:2605–2607. doi: 10.1093/carcin/16.11.2605. [DOI] [PubMed] [Google Scholar]
- 33.Shoemaker A R, Moser A R, Dove W F. Cancer Res. 1995;55:4479–4485. [PubMed] [Google Scholar]
- 34.Albanes D. Cancer Res. 1987;47:1987–1992. [PubMed] [Google Scholar]
- 35.Weindruch R, Albanes D, Kritchevsky D. Hematol Oncol Clin North Am. 1991;5:79–89. [PubMed] [Google Scholar]
- 36.Reddy B S, Wang C X, Maruyama H. Cancer Res. 1987;47:1226–1228. [PubMed] [Google Scholar]
- 37.Hursting S D, Perkins S N, Phang J M. Proc Natl Acad Sci USA. 1994;91:7036–7040. doi: 10.1073/pnas.91.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers A E, Longnecker M P. Lab Invest. 1988;59:729–759. [PubMed] [Google Scholar]
- 39.Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Proc Natl Acad Sci USA. 1995;92:4482–4486. doi: 10.1073/pnas.92.10.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]