Abstract
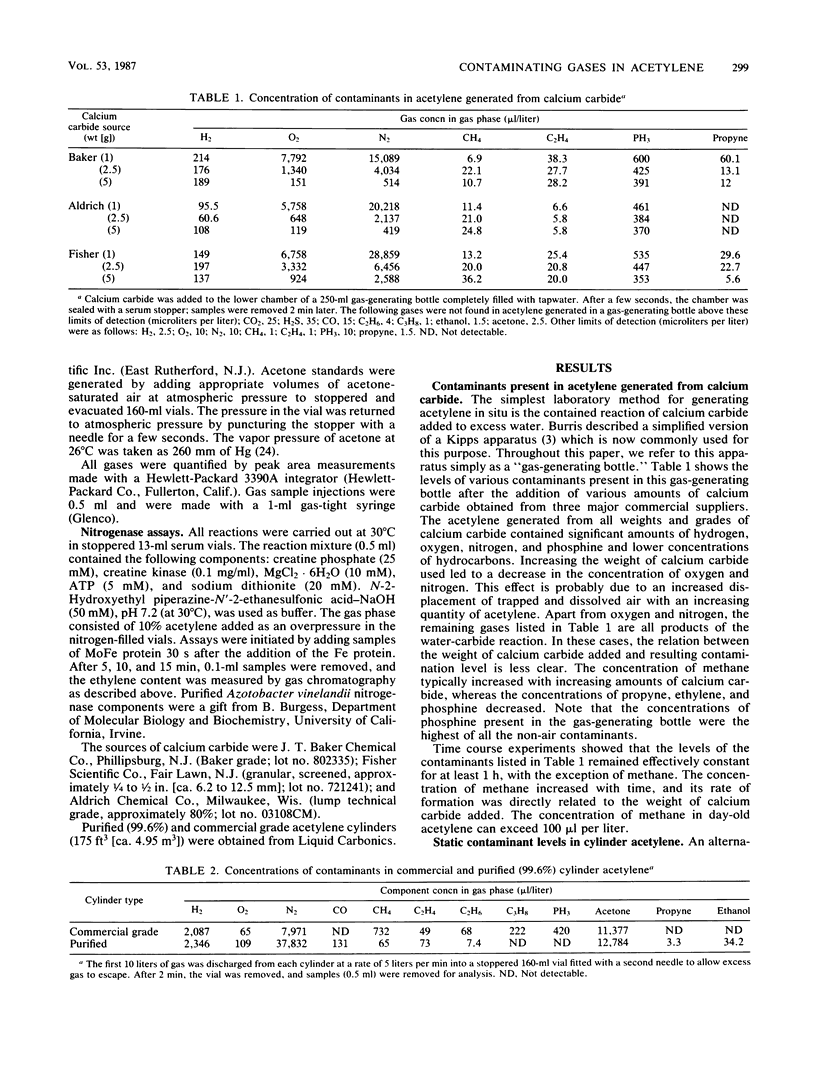
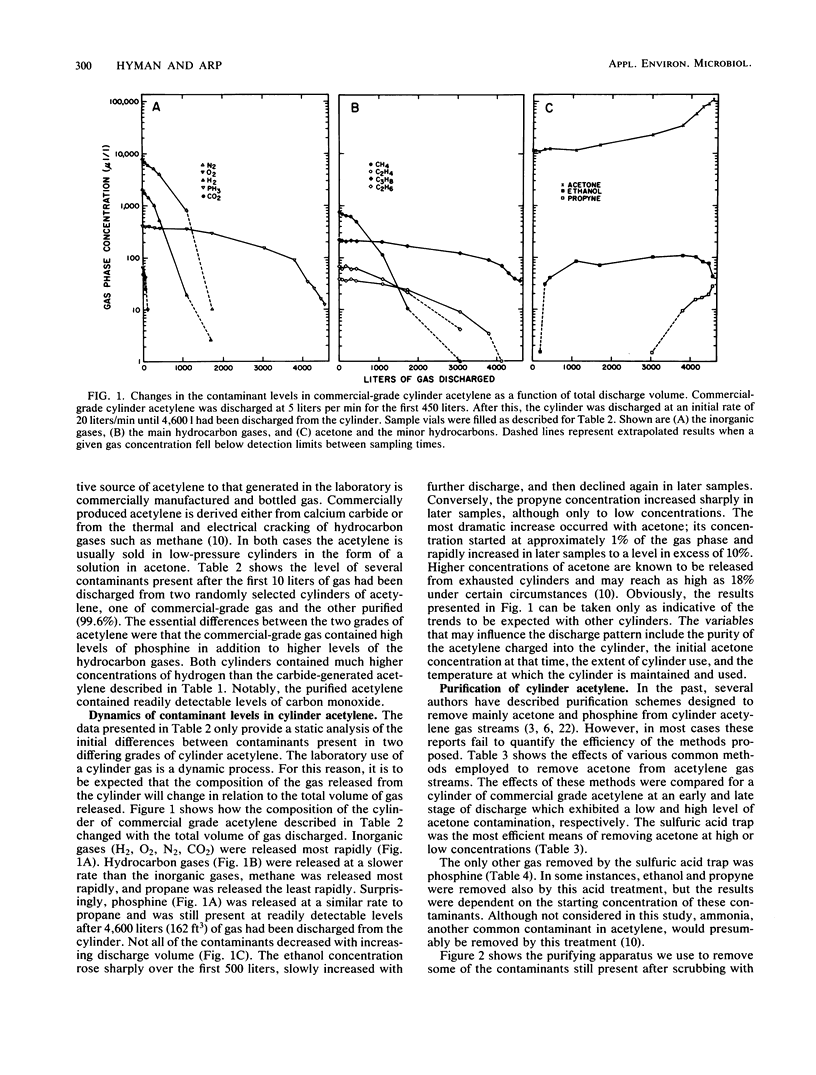
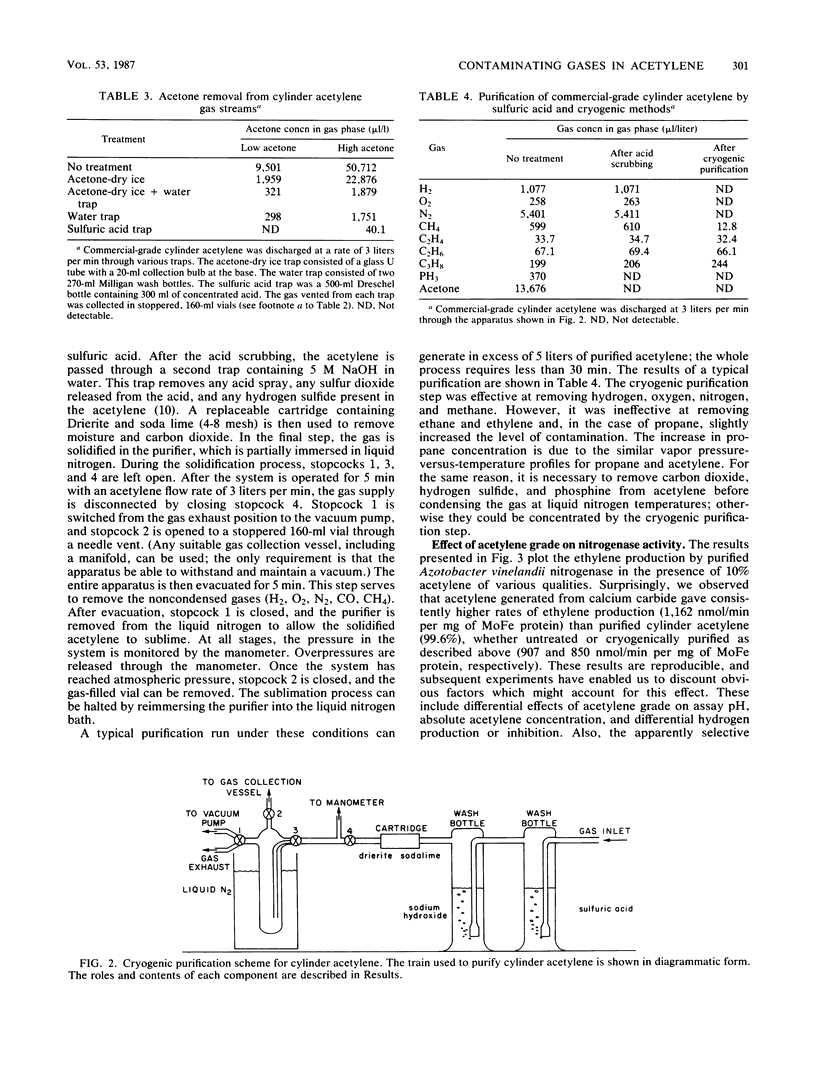
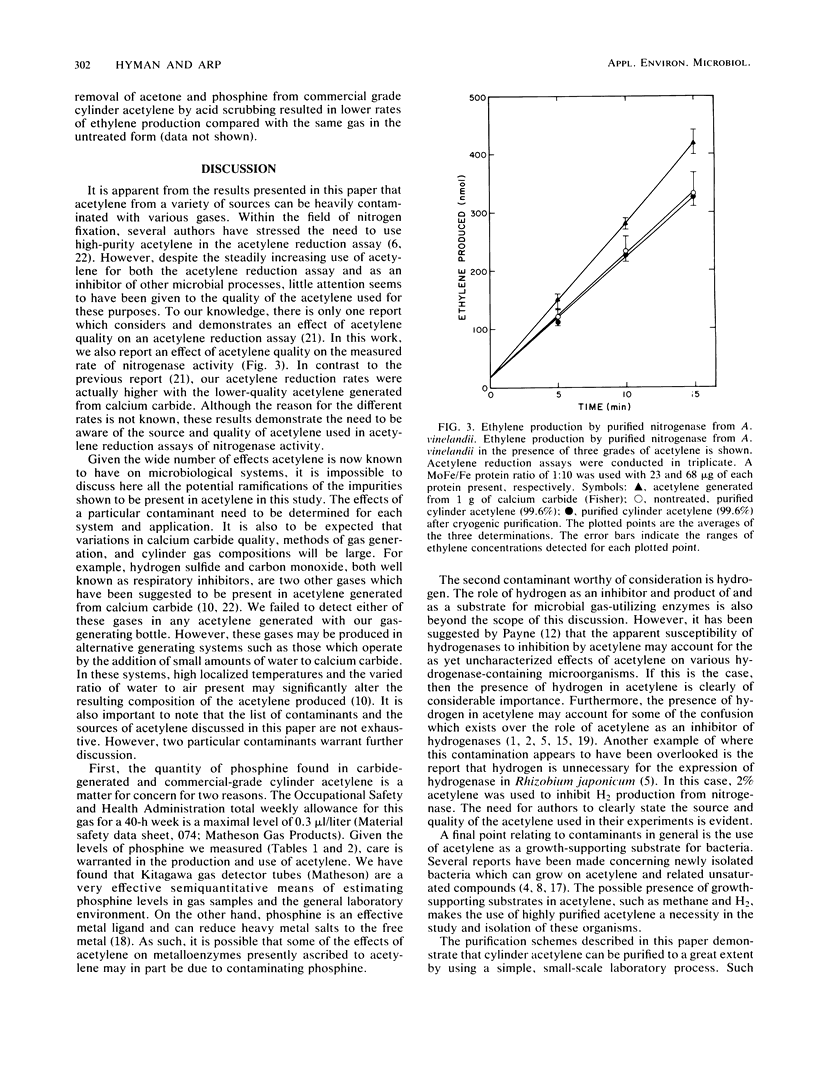
Acetylene generated from various grades of calcium carbide and obtained from commercial- and purified-grade acetylene cylinders was shown to contain high concentrations of various contaminants. Dependent on the source of acetylene, these included, at maximal values, H2 (0.023%), O2 (0.779%), N2 (3.78%), PH3 (0.06%), CH4 (0.073%), and acetone (1 to 10%). The concentration of the contaminants in cylinder acetylene was highly dependent on the extent of cylinder discharge. Several conventional methods used to partially purify cylinder acetylene were compared. A small-scale method for extensively purifying acetylene is described. An effect of acetylene quality on acetylene reduction assays conducted with purified nitrogenase from Azotobacter vinelandii was demonstrated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp D. J., Burris R. H. Kinetic mechanism of the hydrogen-oxidizing hydrogenase from soybean nodule bacteroids. Biochemistry. 1981 Apr 14;20(8):2234–2240. doi: 10.1021/bi00511a025. [DOI] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem J. 1985 May 1;227(3):719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner D., Bartha R. Growth of Nocardia rhodochrous on acetylene gas. J Bacteriol. 1979 Jul;139(1):225–230. doi: 10.1128/jb.139.1.225-230.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J Biol Chem. 1980 Jan 25;255(2):704–707. [PubMed] [Google Scholar]
- Oremland R. S., Taylor B. F. Inhibition of methanogenesis in marine sediments by acetylene and ethylene: validity of the acetylene reduction assay for anaerobic microcosms. Appl Microbiol. 1975 Oct;30(4):707–709. doi: 10.1128/am.30.4.707-709.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Grant M. A. Influence of acetylene on growth of sulfate-respiring bacteria. Appl Environ Microbiol. 1982 Mar;43(3):727–730. doi: 10.1128/aem.43.3.727-730.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. A., Hill S., Yates M. G. Inhibition by acetylene of conventional hydrogenase in nitrogen-fixing bacteria. Nature. 1976 Jul 15;262(5565):209–210. doi: 10.1038/262209a0. [DOI] [PubMed] [Google Scholar]
- Sørensen J. Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. Appl Environ Microbiol. 1978 Feb;35(2):301–305. doi: 10.1128/aem.35.2.301-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


