Abstract
The elucidation of the mechanisms of antigen presentation by major histocompatibility complex class I molecules has stimulated the search for nonreplicative vectors that could deliver CD8+ T cell epitopes to the cytosol of antigen-presenting cells to trigger the activation of specific cytotoxic T lymphocytes (CTLs) in vivo. In the present study, we investigated the potential ability of an invasive adenylate cyclase toxin from Bordetella pertussis, carrying a CD8+ T cell epitope from the nucleoprotein of lymphocytic choriomeningitis virus (LCMV), to stimulate protective anti-viral immunity. Mice immunized with this recombinant toxin developed strong CTL responses against LCMV-infected target cells. Moreover, these mice were protected against an intracerebral challenge with a virulent strain of LCMV that killed all nonimmunized mice within 7 days. This protection was abolished after in vivo elimination of CD8+ T cells. A mutant toxin devoid of adenylate cyclase activity (i.e., cAMP synthesizing activity) was constructed by insertion of a dipeptide into the catalytic site of the molecule. This genetically detoxified invasive toxin carrying the LCMV epitope stimulated a strong CTL response against both peptide-coated and virus-infected target cells, and mice immunized with this molecule were fully protected against a lethal intracerebral LCMV challenge. To our knowledge, this study represents the first demonstration that a genetically detoxified bacterial toxin carrying a viral CTL epitope can stimulate efficient protective immunity.
Anti-viral cytotoxic T lymphocytes (CTLs) recognize short peptides derived from viral proteins that bind to major histocompatibility complex (MHC) class I molecules. Such virus-specific CTLs play a crucial role in the resistance against many viral infections, including influenza virus (1, 2), Sendai virus (3), and lymphocytic choriomeningitis virus (LCMV) (4, 5).
It is now well established that efficient antigen presentation by MHC class I molecules requires the presence of the antigen in the cytosolic compartment of the presenting cells (6). Therefore, in most cases, nonreplicative antigens such as soluble proteins are ineffective for inducing cytotoxic T cell responses, and most CTL activation strategies are based on the use of live vectors. We have recently demonstrated that an invasive and nonreplicative vector, the adenylate cyclase toxin (CyaA) of Bordetella pertussis, can deliver CTL epitopes into the cytosol of eukaryotic cells (7, 8). This toxin, consisting of 1706 amino acid residues, is able to invade a large number of eukaryotic cells and to deliver its N-terminal catalytic domain (400 amino acid residues) directly into the cytosol through the cytoplasmic membrane (9–11). Foreign peptides of up to 16 residues can be inserted into various permissive sites of the catalytic domain of CyaA without altering its main properties such as stability and catalytic and invasive activities (7, 12). Purified CyaA toxins carrying CTL epitopes from the nucleoprotein of LCMV or from the V3 region of HIV-1 gp120 were shown to stimulate strong specific CTL responses, mediated by class I-restricted CD8+ T cells and able to kill target cells coated with the relevant peptide (8). Furthermore, a genetically inactivated recombinant toxin carrying the LCMV epitope also induced strong LCMV-specific CTL responses (8).
These recombinant invasive molecules, therefore, represent attractive new vectors for CTL activation. To demonstrate the potential of this new strategy for eliciting anti-viral immunity, we show in the present study that CyaA molecules carrying an LCMV CD8+ epitope can induce protection against a lethal intracerebral infection with this virus. These results conclusively support the contention that these recombinant toxins represent powerful tools to stimulate protective anti-viral responses.
MATERIALS AND METHODS
Mice, Peptides, and Recombinant Adenylate Cyclase Toxins.
Female BALB/c mice, aged 8–14 weeks, were obtained from Iffa Credo (L’Arbresle, France). The production and purification of recombinant adenylate cyclase toxins have been described (8). The synthetic peptide p118-132 corresponding to the H-2d LCMV CD8+ epitope (RPQASGVYMGNLTAQ)(13) of LCMV nucleoprotein was synthesized by Neosystem (Strasbourg, France).
Immunization of Mice and Virus Challenge Infection.
Mice were immunized intraperitoneally (i.p.) on days 0 and 21 with 50 μg of purified recombinant adenylate cyclase toxins mixed with 1 mg of aluminum hydroxide in phosphate-buffered saline (PBS). Control groups were injected i.p. with PBS or with 105 foci (14) of LCMV (strain Arm/53b). Immunized mice were challenged on days 28 or 63 by intracerebral injection of 101.7 or 103.5 foci of LCMV. To control the viral clearance and the immune state of the surviving mice, they were anesthetized, bled, and sacrificed 21 days after the LCMV challenge. Virus was checked in kidneys by antigen-capture ELISA and serum anti-LCMV IgG levels were tested by ELISA (15).
In vivo depletion of CD4+ or CD8+ T cells was carried out by injections i.p. of rat anti-CD4 (16) or anti-CD8 (17) monoclonal antibodies (mAbs) on days −1, 0, +1, 14, 20, 21, and 22, as described (8). The depletion efficiency was checked by cytofluorometric analysis. Depletion of CD4+ or CD8+ T cells was >95% after treatment with the appropriate mAb.
In Vitro Stimulation of LCMV-Specific CTLs and Cytotoxic Assay.
Stimulation of LCMV-specific CTLs by recombinant toxins has been described (8). In brief, responder spleen cells (2.5 × 107 cells per 10 ml) from in vivo-primed mice were cocultured with 2.5 × 107 syngeneic nonimmunized irradiated cells pulsed-labeled with 1 μM p118-132 peptide. Anti-viral CTLs were generated in control mice by injecting i.p. 105 foci of LCMV, and the splenic CTL response was tested on day 7 (18). For both toxins and LCMV-induced CTLs, a 4-h cytotoxic assay was performed by incubating effector cells with peptide-coated P815 (8) or LCMV-infected J774 (19) target cells, radiolabeled with 51Cr. Nonspecific killing was evaluated on noncoated or noninfected target cells.
The percentage of specific lysis was calculated as follows: (experimental cpm − spontaneous cpm release)/(total cpm − spontaneous cpm release) × 100. Background (medium) was always less than 25% of maximal release. The SD of duplicate wells (killing on peptide-coated target cells) or SD of quadruplicate wells (killing on infected target cells) was always less than 5% of the specific 51Cr release.
RESULTS
Induction of Anti-Viral CTL Responses by Immunization with Recombinant Adenylate Cyclase Toxins of B. pertussis Carrying the LCMV CD8+ T Cell Epitope.
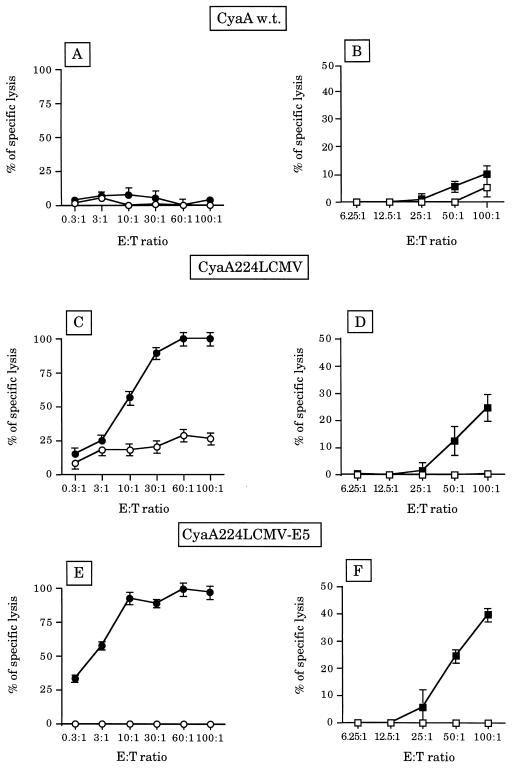
A recombinant adenylate cyclase of B. pertussis with an LCMV nucleoprotein CD8+ T cell epitope (amino acids 118-132) inserted into the catalytic domain of the toxin between residues 224 and 225 (CyaA224LCMV) was expressed in Escherichia coli and purified to homogeneity. BALB/c mice were immunized on days 0 and 21 with 50 μg of CyaA224LCMV in the presence of alum. On day 28, CTL activity was determined after a secondary in vitro stimulation with the p118-132 peptide, by using as target cells either P815 cells coated with the p118-132 peptide or J774 cells infected with LCMV. As shown (8), spleen cells from mice immunized with CyaA224LCMV efficiently killed P815 target cells coated with the p118-132 LCMV synthetic peptide (Fig. 1C), whereas no cytotoxic activity was observed with spleen cells from control mice immunized with the wild-type CyaA (Fig. 1A). More importantly, a significant cytotoxic activity of spleen cells from mice immunized with CyaA224LCMV was also observed against LCMV-infected J774 target cells (Fig. 1D), whereas no cytotoxic activity was observed with spleen cells from mice immunized with the wild-type CyaA. Both cytotoxic activities observed against peptide-coated or LCMV-infected targets were abolished after in vivo depletion of CD8+ lymphocytes, thus showing that this killing was mediated by CD8+ cytotoxic T cells (data not shown).
Figure 1.
Induction of LCMV-specific CTLs after immunization with CyaA224LCMV or CyaA224LCMV-E5. On days 0 and 21, BALB/c mice were injected with 50 μg of wild-type CyaA (A and B), CyaA224LCMV (C and D), or CyaA224LCMV-E5 (E and F) mixed with 1 mg of alum. Seven days later, spleens were harvested and cell suspensions were restimulated in vitro with peptide p118-132 (1 μg/ml) in the presence of irradiated syngenic spleen cells. After 5 days in culture, harvested cells were assayed for the presence of peptide or virus-specific cytolytic T cells in a standard 4-h 51Cr-release assay using the following cells. (A, C, and E) P815 target cells incubated with medium alone (○) or with peptide p118-132 (•). (B, D, and F) J774 target cells incubated with medium alone (□) or infected with LCMV (▪). Control LCMV-immune mice showed high levels of cytotoxic activity [50–60% of lysis at a 50:1 effector/target (E:T) ratio].
We also analyzed the CTL responses of mice immunized with a genetically detoxified variant of CyaA224LCMV that is devoid of adenylate cyclase activity but still retains its invasive properties (8). BALB/c mice immunized with this detoxified molecule, CyaA224LCMV-E5, developed a strong CTL activity against peptide-coated target cells (Fig. 1E). Moreover, spleen cells from these mice also killed LCMV-infected target cells very efficiently (Fig. 1F), showing that the catalytic activity of the toxin is not required for the induction of virus-specific CTL responses.
Recombinant Adenylate Cyclase Toxins Carrying the LCMV CD8+ T Cell Epitope Protect Mice Against a Lethal LCMV Infection.
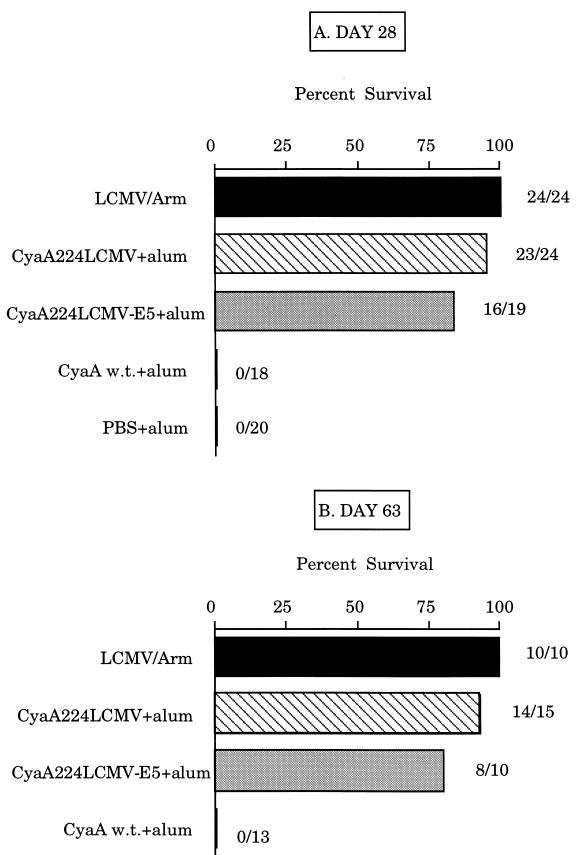
To determine whether CTL responses stimulated by recombinant CyaA toxins could confer protection against LCMV infection, BALB/c mice were immunized with 50 μg of CyaA224LCMV or CyaA224LCMV-E5 in the presence of alum on days 0 and 21. They were then challenged intracerebrally with 101.7 foci of the virulent Armstrong strain of LCMV (LCMV-Arm) on day 28 (Fig. 2A) or day 63 (Fig. 2B). It is important to note that intracerebrally inoculated LCMV-Arm provokes an acute lethal infection in BALB/c mice (20), whereas intraperitoneal inoculation causes only a transient infection characterized by a strong and long-lasting protective immune response (21).
Figure 2.
Immunization with CyaA224LCMV or CyaA224LCMV-E5 induces full protection against a lethal LCMV challenge. On days 0 and 21, BALB/c mice were immunized with 50 μg of wild-type CyaA (w.t.), CyaA224LCMV, or CyaA224LCMV-E5 mixed with 1 mg of alum. Control groups received either PBS or 105 foci of LCMV i.p. On day 28 (A) or on day 63 (B), mice were challenged intracerebrally with 101.7 foci of LCMV. Mortality was monitored for 21 days, at which time the mice were sacrificed for analysis of serum levels of anti-LCMV antibodies and the presence of viral antigens in the kidneys. The percent of surviving mice and the number of surviving mice out of the total number of challenged mice are shown for each group.
After immunization with CyaA224LCMV, mice challenged on day 28 showed very good protection (96% survival) against an intracerebral injection of 101.7 foci of LCMV, which killed all control mice in 7 days. A similar degree of protection (93%) was observed when the LCMV challenge was done on day 63, thus, confirming that CyaA224LCMV induces a long-lasting LCMV-specific CTL response (8). As expected, all control mice injected with the wild-type CyaA or PBS alone died of choriomeningitis in the 7 days after LCMV intracerebral inoculation. Interestingly, a very efficient protection was also observed in mice immunized with the detoxified CyaA224LCMV-E5. After intracerebral challenge on day 28 or 63, 84% and 80% survival, respectively, of immunized mice was obtained.
To verify that LCMV had been eliminated from protected animals, survivors were sacrificed 21 days after challenge and the kidneys were examined for the presence of viral antigens by antigen-capture ELISA. No virus was detected in any of the vaccinated animals (data not shown), thus showing that vaccination with these recombinant toxins, including the genetically detoxified molecule, provided a full protection against LCMV infection. Furthermore, all surviving animals had high anti-LCMV antibody titers, indicating that they had developed a full immunity against LCMV.
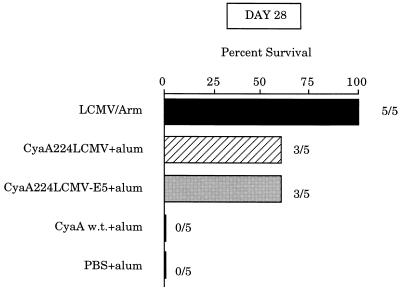
We next evaluated the protective effect of recombinant adenylate cyclase carrying the LCMV epitope by examining the resistance of mice to a higher dose of virus. After two immunizations on days 0 and 21 with wild-type CyaA, CyaA224LCMV, or CyaA224LCMV-E5, mice were challenged on days 28 or 63 with 103.5 foci of LCMV injected intracerebrally. As shown in Fig. 3, a partial protection was observed in the groups of mice immunized with either the CyaA224LCMV or CyaA224LCMV-E5 and challenged on day 28. The surviving mice had eliminated the virus from the kidneys on day 21 after challenge and had raised high titers of LCMV-specific antibodies (data not shown). Consequently, these results show that complete protection with total viral clearance was achieved.
Figure 3.
Immunization with CyaA recombinant toxins partially protects mice against a lethal LCMV challenge with a high dose of virus. On days 0 and 21, BALB/c mice were immunized with 50 μg of wild-type CyaA (w.t.), CyaA224LCMV, or CyaA224LCMV-E5 mixed with 1 mg of alum. On day 28, mice were challenged intracerebrally with 103.5 foci of LCMV. Control groups received either PBS or 105 foci of LCMV i.p. Mortality was monitored for 21 days at which time mice were sacrificed for analysis of serum levels of anti-LCMV antibodies and the presence of viral antigens in the kidneys. The percent of surviving mice and the number of surviving mice out of the total number of challenged mice are shown for each group.
However, when the challenge was performed on day 63, 6 weeks after the last immunization, all the mice died, except control animals previously immunized by an i.p. injection of LCMV-Arm virus (data not shown). As observed in other experimental models (22, 23), mice immunized with recombinant adenylate cyclases died earlier than PBS injected mice (mean survival time of 4.5 instead of 7 days for control mice) with typical clinical symptoms of choriomeningitis.
In Vivo Depletion of CD8+ T Cells Abrogates the Protection Conferred by the Recombinant Adenylate Cyclase Toxins.
Since we have shown (8) that the 118-132 LCMV epitope contains both a CD4+ and a CD8+ CTL epitope, it was necessary to determine whether the protection induced by immunization with the recombinant adenylate cyclase was mediated by CD4+ or CD8+ T cells. To address this point, we specifically depleted these T cell subpopulations with specific mAbs as described (8). Mice were repeatedly injected i.p. with rat mAbs specific for CD4+ or CD8+ T cells and immunized with CyaA224LCMV or CyaA224LCMV-E5. In vivo administration of these antibodies resulted in depletion of more than 95% of circulating CD4+ or CD8+ T cells, as determined by flow cytofluorometry (data not shown).
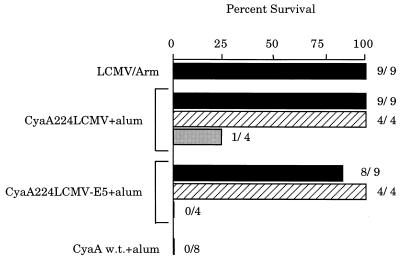
As shown in Fig. 4, all mice immunized with LCMV-Arm, CyaA224LCMV, or CyaA224LCMV-E5 survived an intracerebral challenge with LCMV, whereas control mice injected with wild-type CyaA were killed when inoculated with the virus. However, LCMV inoculation killed CyaA224LCMV- or CyaA224LCMV-E5-immunized mice that had been treated with the anti-CD8+ T cell mAbs. In contrast, in vivo depletion of CD4+ T cells did not modify the protection induced by the recombinant toxins. These results therefore demonstrate that the protective anti-LCMV immunity induced by these recombinant adenylate cyclase toxins is mediated exclusively by CD8+ T cells.
Figure 4.
In vivo depletion of CD8+ T cells abrogates protection of mice immunized with recombinant CyaA224LCMV or CyaA224LCMV-E5. On days −1, 0, +1, 14, 20, 21, and 22, BALB/c mice received i.p. injections of 0.3 mg of anti-CD4 (hatched bars) or anti-CD8 (shaded bars) mAb or of PBS (solid bars). On days 0 and 21, mice were injected with 50 μg of CyaA224LCMV or CyaA224LCMV-E5 in the presence of 1 mg of alum. Control groups were immunized i.p. with wild-type CyaA or with LCMV but did not receive further treatment. On day 28, mice were challenged by intracerebral injection of 101.7 foci of LCMV. Mortality was monitored for 21 days at which time mice were sacrificed for analysis of serum levels of anti-LCMV antibodies and the presence of viral antigens in the kidneys. The percent of surviving mice and the number of surviving mice out of the total number of challenged mice are shown for each group.
DISCUSSION
To our knowledge, this study represents the first demonstration that a bacterial toxin carrying a viral CD8+ T cell epitope can induce protective immunity against a lethal virus infection in mice. Several model systems involving nonreplicative recombinant toxins have been tested for induction of CD8+ CTL responses. Modified Pseudomonas exotoxins carrying peptide epitopes from influenza matrix protein and nucleoprotein have been shown in vitro to be internalized and efficiently presented by MHC class I-positive target cells (24, 25). However, the in vivo immunogenicity of these molecules remains to be established. More recently, the protective antigen binding domain of anthrax lethal factor fused to a heterologous protein was shown to retain its ability to complement the protective domain in mediating translocation of the protein to the cytoplasm (26). However, use of this model for vaccination has not been documented.
In our approach, we have chosen the adenylate cyclase from B. pertussis since this toxin has the ability to deliver its catalytic domain to a large variety of target cells, where it actively synthesizes supraphysiological levels of cAMP. Foreign epitopes, inserted into the catalytic domain of the toxin, could thus be delivered to the cytosol of the antigen-presenting cells and enter the MHC class I presentation pathway. We have provided (8) strong evidence that the ability of recombinant CyaA to induce CTL responses is dependent upon its capacity to penetrate target cells independently of the adenylate cyclase enzymatic activity. Indeed, a truncated recombinant molecule that carries the LCMV epitope but lacks invasive activity was unable to stimulate CTL responses (8). In vitro, we also showed that a LCMV CD8+ T cell epitope, carried by recombinant CyaA, could be delivered to MHC class I molecules (7). The present data confirm these results and also demonstrate that cytotoxic T cells stimulated by the recombinant toxin are able to recognize target cells infected with the virus. Hence, the fine specificity of these T cells allows them to recognize the naturally processed LCMV epitope. More importantly, mice immunized with recombinant CyaA carrying the LCMV epitope are fully protected against a lethal challenge with the Armstrong strain of LCMV. This virus strain provokes an acute lethal infection in BALB/c mice when inoculated intracranially, whereas when inoculated intraperitoneally, mice become only transiently infected and show a strong and long-lasting protection. It should be noted that all immunized mice that survived the LCMV challenge had eliminated the virus from the kidneys 3 weeks after inoculation. This last result shows that a complete protection associated with total viral clearance, and not a carrier state, was achieved after immunization with recombinant adenylate cyclase.
It has been shown that viral clearance and protection from LCMV disease requires a potent LCMV-specific CTL response (22). Indeed, various live vectors, such as vaccinia (22), listeria (19, 27), or Mengo virus (15), expressing the nucleoprotein gene were previously shown to stimulate specific CTL responses and to induce protection against a lethal LCMV infection. Recently, the intramuscular injection of plasmid DNA encoding the LCMV nucleoprotein was also shown to protect mice against a lethal LCMV infection, but this protection was incomplete and only 50% of immunized BALB/c mice survived after challenge (28). Moreover, another study demonstrated that mice immunized with DNA encoding the nucleoprotein gene displayed enhanced immunopathology leading to accelerated mortality after intracranial challenge with a high dose of virus (23). An exacerbation of the disease was also observed in mice immunized with Listeria monocytogenes expressing the LCMV nucleoprotein and challenged 2–5 weeks after vaccination. This phenomenon is explained by an impaired balance between the virus titers in the choriomeninges and the intensity of the T cell response (22). Such enhanced immunopathology was also observed in our study when mice were challenged with a high dose of virus 2 months after immunization, indicating that the protective immunity induced by the recombinant toxins declined too rapidly to effectively clear high titers of viral particles. Therefore, it seems important to increase the duration of the CTL memory response stimulated by such recombinant toxins or to stimulate more CTL precursors. Various approaches to increase the efficiency of these CyaA vectors, such as inserting several copies of the LCMV epitope, are currently under evaluation.
The results of the present study clearly confirm the strong potential of these invasive molecules to trigger CTL responses against viral epitopes. Recombinant CyaA molecules can be easily produced in E. coli, and their large-scale purification to homogeneity from inclusion bodies can be achieved by simple procedures. The fact that a genetically detoxified molecule retains its capacity to stimulate a fully protective anti-LCMV immune response offers attractive perspectives for the use of these molecules in the development of safe nonreplicative anti-viral vaccines.
Acknowledgments
We thank Jeanine Foulon and Antonio Membrillero-Pizzaro for excellent technical assistance, Servanne Pires for typing the manuscript, and D.S. Longacre-Andre for correcting the English of the manuscript. This work was supported by grants from the Centre National de la Recherche Scientifique (URA1129) and from the Human Science Frontier Program Organization.
ABBREVIATIONS
- CTL
cytotoxic T lymphocyte
- LCMV
lymphocytic choriomeningitis virus
- MHC
major histocompatibility complex
References
- 1.Lin Y L, Askonas B A. J Exp Med. 1981;154:225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor P M, Askonas B A. Eur J Immunol. 1983;13:707–711. doi: 10.1002/eji.1830130904. [DOI] [PubMed] [Google Scholar]
- 3.Kast W M, Bronkhorst A M, De Waal L P, Melief C J M. J Exp Med. 1986;164:723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne J A, Oldstone M B A. J Virol. 1984;51:682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann-Grube F, Mosphokidis D, Lohler J. Ann NY Acad Sci. 1988;532:238–256. doi: 10.1111/j.1749-6632.1988.tb36343.x. [DOI] [PubMed] [Google Scholar]
- 6.Germain R N. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 7.Sebo P, Fayolle C, d’Andria O, Ladant D, Leclerc C, Ullmann A. Infect Immun. 1995;63:3851–3857. doi: 10.1128/iai.63.10.3851-3857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayolle C, Sebo P, Ladant D, Ullmann A, Leclerc C. J Immunol. 1996;156:4697–4706. [PubMed] [Google Scholar]
- 9.Hanski E. Trends Biochem Sci. 1989;14:459–463. doi: 10.1016/0968-0004(89)90106-0. [DOI] [PubMed] [Google Scholar]
- 10.Gordon V M, Young W W, Jr, Lechler S M, Gray M C, Leppla S H, Hewlett E L. J Biol Chem. 1989;264:14792–14796. [PubMed] [Google Scholar]
- 11.Mock M, Ullmann A. Trends Microbiol. 1993;1:187–192. doi: 10.1016/0966-842x(93)90089-a. [DOI] [PubMed] [Google Scholar]
- 12.Ladant D, Glaser P, Ullmann A. J Biol Chem. 1992;267:2244–2250. [PubMed] [Google Scholar]
- 13.Aichele P, Hengartner H, Zinkernagel R M, Schulz M. J Exp Med. 1990;171:1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel R M. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 15.Altmeyer R, Girard M, van der Werf S, Mimic V, Seigneur L, Saron M F. J Virol. 1995;69:3193–3196. doi: 10.1093/benz/9780199773787.article.b00034516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierres M, Goridis C, Goldstein P. Eur J Immunol. 1982;12:60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- 17.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loker M R, Pierres M, Fitch F W. J Immunol. 1984;131:2445–2451. [PubMed] [Google Scholar]
- 18.Zinkernagel R M, Doherty P C. Scand J Immunol. 1974;3:287–294. doi: 10.1111/j.1365-3083.1974.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 19.Goossens P L, Milon G, Cossart P, Saron M F. Int Immunol. 1995;7:797–805. doi: 10.1093/intimm/7.5.797. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann-Grube F. Arch Virusforsch. 1964;14:344–350. doi: 10.1007/BF01555827. [DOI] [PubMed] [Google Scholar]
- 21.Kimmig W, Lehmann-Grube F. J Gen Virol. 1979;45:703–710. doi: 10.1099/0022-1317-45-3-703. [DOI] [PubMed] [Google Scholar]
- 22.Oehen S, Hengartner H, Zinkernagel R M. Science. 1991;251:195–198. doi: 10.1126/science.1824801. [DOI] [PubMed] [Google Scholar]
- 23.Zarozinski C C, Fynan E F, Selin L K, Robinson H L, Welsh R M. J Immunol. 1995;154:4010–4017. [PubMed] [Google Scholar]
- 24.Donnelly J J, Ulmer J B, Hawe L A, Friedman A, Shi X P, Leander K R, Shiver J W, Oliff A I, Martinez D, Montgomery D, Liu M A. Proc Natl Acad Sci USA. 1993;90:3530–3534. doi: 10.1073/pnas.90.8.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulmer J B, Donnelly J J, Liu M A. Eur J Immunol. 1994;24:1590–1596. doi: 10.1002/eji.1830240721. [DOI] [PubMed] [Google Scholar]
- 26.Milne J C, Blanke S R, Hanna P C, Collier R J. Mol Microbiol. 1995;15:661–666. doi: 10.1111/j.1365-2958.1995.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 27.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokohama M, Zhang J, Whitton J L. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]