Abstract
Multiple endocrine neoplasia type 2 (MEN 2) is a dominantly inherited cancer syndrome that comprises three clinical subtypes: MEN type 2A (MEN-2A), MEN type 2B (MEN-2B), and familial medullary thyroid carcinoma (FMTC). Medullary thyroid carcinoma (MTC), a malignant tumor arising from calcitonin-secreting thyroid C cells, is the cardinal disease feature of this syndrome, and mortality in affected MEN-2 patients is mainly caused by this malignancy. Germ-line mutations of the RET protooncogene, which encodes a receptor tyrosine kinase, are responsible for these three neoplastic-prone disorders. MEN2 mutations convert the RET protooncogene in a dominantly acting oncogene as a consequence of the ligand-independent activation of the tyrosine kinase. The majority of MEN2A and FMTC mutations are located in the extracellular domain and cause the replacement of one of five juxtamembrane cysteines by a different amino acid. To examine whether expression of a MEN2A allele of RET results in transformation of C cells, we have used the transgenic approach. Expression of the RET gene altered by a MEN2A mutation was targeted in C cells by placing the transgene under the control of the calcitonin gene-related peptide/calcitonin promoter. Animals of three independent transgenic mouse lines, which expressed the transgene in the thyroid, displayed overt bilateral C cell hyperplasia as early as 3 weeks of age and subsequently developed multifocal and bilateral MTC. Moreover, these tumors were morphologically and biologically similar to human MTC which afflicts MEN- 2 individuals. These findings provide evidence that the MEN2A mutant form of RET is oncogenic in parafollicular C cells and suggest that these transgenic mice should prove a valuable animal model for hereditary MTC.
Medullary thyroid carcinoma (MTC) is a malignant tumor arising from calcitonin (CT)-secreting parafollicular C cells that may occur sporadically or as a component of the familial cancer syndrome, multiple endocrine neoplasia type 2 (MEN-2) (1, 2). MEN 2 comprises three related dominantly-inherited disorders: MEN type 2A (MEN-2A), type 2B (MEN-2B), and familial MTC (FMTC). MEN-2A is characterized by the association of MTC, pheochromocytomas, and hyperparathyroidism, while MEN-2B includes MTC, pheochromocytoma, neuromas of the lips, tongue and conjunctivae, intestinal ganglioneuromatosis, and skeletal abnormalities (1, 2). In FMTC, the medullary thyroid carcinoma is the sole clinical manifestation (1, 2).
Germ-line mutations of the RET protooncogene, which is located in the pericentromeric region of chromosome 10 (10 q11.2) (3), are responsible for most cases of MEN-2 (4–9). The RET gene encodes a transmembrane tyrosine kinase protein. Three RET isoforms that differ by 9, 43, and 51 aa in the carboxyl-terminal domain (here referred to as RET9, RET43, and RET51) are encoded by alternative spliced mRNAs (10, 11). Although the function of the RET protein is not completely understood, the phenotype of the mice homozygous for a null mutation in the RET gene has provided several clues to the biological role of RET. These mice display kidney agenesis or dysgenesis and lack intestinal autonomic ganglia, thus indicating that RET is essential for both renal development and during enteric neurogenesis (12). Several recent studies have demonstrated that the RET protein is a receptor for the glial cell-line derived neurotrophic factor (GDNF) (13–16). However, biochemical analyses support the notion that the binding of GDNF requires the assembly of a multisubunit complex that includes not only RET but also a glycosylphosphatidylinositol-linked membrane protein named GDNFR-α (15, 16). Evidence that GDNF is a RET ligand has been corroborated by the demonstration that mice homozygous for a disruptive mutation in the RET gene exhibit a phenotype similar to mice null for GDNF (17–19).
In MEN-2A and FMTC, mutations primarily involve five cysteines clustered in the extracytoplasmic domain (Cys-609, -611, -618, -620, and -634), and these alterations result in the substitution of a single cysteine by a different amino acid (4, 5, 20, 21). The great majority of MEN2A mutations affects codon 634, while in FMTC the mutations are equally distributed among codons 618, 620, and 634. This latter finding indicates that individuals with a germ-line mutation at codon 634 are also at risk for the development of pheochromocytoma and hyperparathyroidism in addition to MTC (20, 21). Recently, two missense mutations located in the sequence coding for the tyrosine kinase domain of RET have been identified in the germ line of six FMTC families (22–24). These two mutations result in the substitution of either Glu-768 or Val-804 by an aspartic acid and a leucine respectively (22, 23). Finally, a unique point mutation that leads to the replacement of a methionine for a threonine (M918T) within the tyrosine kinase domain has been detected in almost all MEN-2B cases (6–9).
All MEN2 mutations tested so far convert the RET protooncogene into a dominantly acting transforming gene. Both MEN2A/FMTC and MEN2B mutations lead to a ligand-independent stimulation of the tyrosine kinase (25–28) through different mechanisms of activation. In the case of MEN-2A/FMTC, mutations of cysteine cause aberrant dimerization of RET via the formation of a disulfide-bonded homodimer (25, 26), while in MEN-2B, the mutation M918T appears to modify the substrate specificity of the tyrosine kinase (26, 29).
Although MEN-2 mutant forms of the RET protein efficiently transform fibroblasts (25–28), their biological effects in thyroid C cells have remained elusive. To address this question we have constructed transgenic mice carrying the RET gene altered by a MEN2A mutation fused to the CT gene-related peptide (CGRP)/CT promoter (30). We report here that early diffuse hyperplasia of thyroid C cells, followed by the onset of multifocal and bilateral MTC, developed in animals from three transgenic mouse lines.
MATERIALS AND METHODS
Construction of the Transgene.
Using site-directed mutagenesis (Transformer Site-Directed Mutagenesis kit; CLONTECH), we introduced a missense mutation into the cDNA coding for the human RET isoform of 1072 aa (28). This mutation (TGC → CGC) results in the substitution of Cys-634 for Arg. The 3290-bp RET cDNA with the mutation at codon 634 was released from the pBluescript SK- (Stratagene) (28) by digestion with HindIII, blunted with Klenow enzyme, and ligated into a pGEM3-based vector (Promega). This vector was composed of the rat CGRP/CT promoter (30) and was flanked 3′ of the cloning site by the intron from the simian virus 40 (SV40) small T antigen and by the cleavage/polyadenylylation signal from the early transcription unit of SV40. The CGRP/CT sequence contained 100 bp of the first exon which is not coding. In this study, we used a slightly longer CGRP/CT promoter than described by Baetscher et al. (30), which comprised 2 kb of 5′ upstream sequence.
Generation of Transgenic Mice.
Pronuclei of fertilized oocytes from (C57BL6 × DBA2)F1 mice were injected with the NheI linearized transgene at a concentration of 2 μg/ml in TE buffer (10 mM Tris·Cl, pH 7.5/0.2 mM EDTA). Eggs surviving microinjection were transferred into the oviducts of pseudopregnant (C57BL6 × CBA) females. DNA was extracted from tail biopsies, digested with BamHI and analyzed by Southern blotting. The probe used was a 2-kb BamHI–BamHI fragment corresponding to a fused hybrid portion of the transgene. This fragment encompassed the 3′ coding sequence of RET plus the intron and the poly(A) cassette of SV40. The probe was labeled with [α-32P] dCTP (3000 Ci/mmol; 1 Ci = 37 GBq) by random priming and standard procedures were applied for hybridization. Lines of transgenic mice were established by crossing each founder mouse with DBA2 mice to obtain the F1 generation, and then the transgenic lines were propagated by breeding the littermates.
Analysis of Transgene Expression.
Thyroid gland and other organs were removed under general anesthesia and polyadenylylated RNA was isolated (Quick-Prep Micro mRNA purification kit; Pharmacia). RNAs were reverse-transcribed with the Moloney murine leukemia virus reverse transcriptase for 60 min at 37°C (first-strand cDNA synthesis kit; Pharmacia) and the resulting cDNA was PCR amplified with Taq DNA polymerase (Promega). The forward primer (5′-GCACAGGAGCCGCTGCCCAGATCAAGAGTCAC-3′) annealed to the first exon of the rat CT promoter. The reverse primer (5′-CAGGAGCTATGGTCCAGGCTCCGGTTAAGG-3′) hybridized to the RET sequence. The PCR conditions included an initial denaturation step at 95°C for 3 min, followed by 30 cycles at 94°C for 1 min, 57°C for 1 min, 72°C for 1 min, and a final extension step at 72°C for 5 min. The size of the amplified fragment was 460 bp. The reaction products were analyzed by electrophoresis on 2% agarose gel.
Histology and Immunohistochemistry.
Thyroid glands were removed under a dissecting microscope. Organs were prepared for histopathological examination by fixation in 10% (vol/vol) formaldehyde and embedded in paraffin. To examine the complete thyroid, serial sections were cut to a thickness of 5 μm and processed either for morphological hematoxylin/eosin or for specific immunohistochemical CT detection. For immunostaining of CT, the peroxydase antiperoxydase method was performed using a rabbit anti-CT serum purchased from Dako. The polyclonal antiserum (used diluted 1:500) recognized processed and unprocessed CT but did not cross-react with CGRP.
CT Assay.
Animals were fasted overnight and blood samples were collected by venous orbital puncture under general anesthesia. Sera were rapidly frozen until analysis. CT levels were measured by standard radioimmunoassay (Calcitonin II, 125I RIA Kit; Instar, Stillwater, MN). To avoid enzymatic degradation of CT in the serum, the whole assay was performed on ice. For the response to i.v. calcium challenge, mice were injected with 100 μl of CaCl2, containing 16 μg of Ca2+/g body weight, and blood was collected 4 min after the beginning of the injection (31). Control data were obtained from nontransgenic mice of the same background, sex, and age as the transgenic mice tested.
Protein Analysis.
NIH 3T3 cells were infected with recombinant retroviruses expressing the human RET9 carrying a mutation at codon 634 (C634R), and a mass population of infected cells was obtained by selecting with puromycin (28). Tissues were lysed in immunoprecipitation buffer (20 mM Tris·HCl, pH 7.8/150 mM NaCl/1% Nonidet P-40/2 mM EDTA/1 mM phenylmethylsulfonyl fluoride/10 μg/ml aprotinin/10 μg/ml leupeptin), incubated on ice for 15 min, and clarified by centrifugation. Protein samples were immunoprecipitated overnight at 4°C with an anti-RET rabbit polyclonal serum raised against a peptide corresponding to the 20 aa of the carboxyl terminus of the short RET isoform (28). Immune complexes were absorbed on protein A coupled to Sepharose beads (protein A Sepharose CL4B; Pharmacia) and successively washed twice in immunoprecipitation buffer and twice in immunokinase assay buffer (50 mM Hepes, pH 7.2/5 mM MnCl2/1 mM phenylmethylsulfonyl fluoride). The immunoprecipitates were then incubated for 20 min at room temperature in 30 μl of immunokinase buffer containing 4 μCi of [γ-32P]ATP (5000 Ci/mmol; ICN) diluted with unlabeled ATP to a final concentration of 26 pmol. The reaction was terminated by addition of Laemmli buffer (20 mM Tris·Cl, pH 6.8/2 mM EDTA/2% SDS/20% glycerol/20 μg/ml of bromophenol blue) omitting the 2-mercaptoethanol. Proteins were boiled 3 min and separated on a SDS/6% polyacrylamide gel.
RESULTS
Generation of Transgenic Mice.
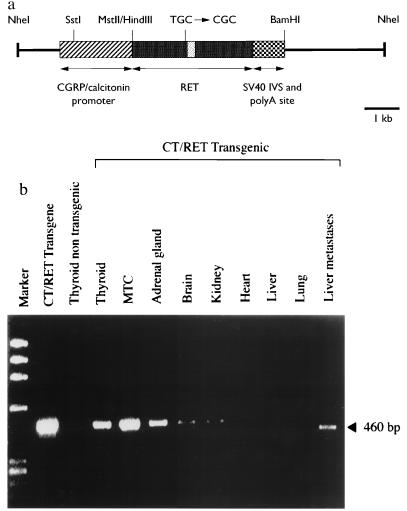
The most common mutation in MEN-2A (≈52% of all cases) occurs at codon 634 and results in the substitution of Cys-634 for an arginine (TGC → CGC) (24). Although mutations at codon 634 are present in 30% of FMTC cases, the mutation C634R has not yet been described in any FMTC cases compiled by the International RET Mutation Consortium (24). The cDNA coding for the human RET9 isoform was subcloned into pBluescript SK- (Stratagene) (28). The mutation that changed Cys-634 → Arg was introduced by site-directed mutagenesis using the unique site-elimination technique. It has been previously shown that the 1.7-kb sequence flanking the first exon of the CGRP/CT gene targets the expression of the transgene to CGRP-containing neurons and to CT-secreting C cells (30). Therefore, to direct expression of the transgene in thyroid C cells, we subcloned the cDNA coding for RET9 carrying the mutation at codon 634 into a vector containing the rat CGRP/CT promoter (30) and a genomic fragment composed of the intron from the SV40 small T antigen and of the cleavage/polyadenylylation signal from the early transcription unit of SV40 (Fig. 1a). Four founder mice (5319, 5322, 5378, 5379), which transmitted the transgene in a Mendelian fashion and carried from 1 to 10 copies of the transgene (data not shown), were bred as lines. Expression of the RET transgene was examined by RT-PCR with primers designed to specifically identify the RET transgene mRNA (Fig. 1b). RT-PCR analyses were performed in at least two F1 transgenic mice per line. In three out of the four lines (5319, 5322, 5379), the transcript corresponding to the transgene was detected in the thyroid of transgenic animals but not in the thyroid of nontransgenic littermates (Fig. 1b). The RET transgene was also expressed, albeit not consistently among the three lines, in the adrenal glands (5319, 5322), in kidneys (5319), and in the brain (5319) (Fig. 1b). Expression was not observed in lung, heart, liver, skin, or gut of animals from the three transgenic lines (Fig. 1b).
Figure 1.
(a) Schematic diagram of the CGRP/CT RET transgene. The rat CT/CGRP promoter drives expression of a human RET cDNA which encodes the short isoform of 1072 aa. A mutation at codon 634 (TGC → CGC) was introduced in the RET cDNA that results in the replacement of the Cys-634 for an arginine. The filled dot box represents the RET transmembrane coding region. (b) Reverse transcription–PCR (RT-PCR) analysis of the expression of the transgene. RNAs were reverse transcribed and the resulting cDNAs were PCR amplified using primers that annealed to the first exon of the rat CT promoter and to the RET sequence. Amplification of a 460-bp PCR product was detected in thyroid, MTC, adrenal gland, brain, and kidney, but not in heart, liver, and lung of mice from the transgenic line 5319 (CT/RET). The transgene transcript was also detected in the hepatic metastatic nodules of one transgenic animal (line 5319).
Thyroid Pathology in Transgenic Mice.
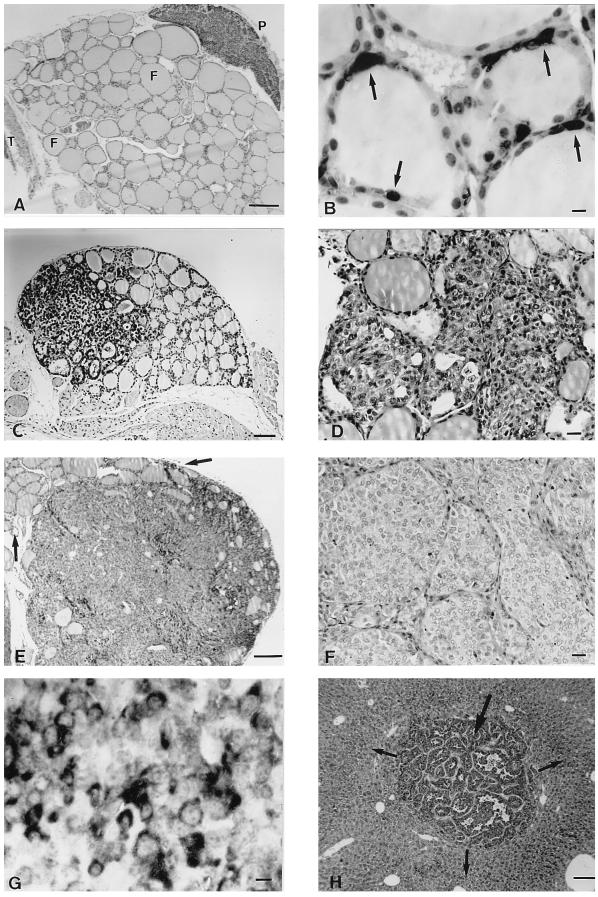
Overall, 45 transgenic mice were examined (age 3 weeks to 24 months) and 42 animals of the three transgenic lines developed bilateral C cell hyperplasia (CCH) and subsequently MTC. Diffuse CCH is characterized by both an increased number of follicles with C cells and the number of C cells per follicle, whereas accumulation of C cells between the follicular epithelium and the basal lamina typifies nodular CCH. Animals of the 5319 mouse line displayed CCH as early as 3 weeks of age (Fig. 2C) and all transgenic mice sacrificed between 2 and 7 months of age demonstrated nodular CCH and/or microscopic foci of MTC (Fig. 2D). After 8 months of age, bilateral and multicentric MTC were unambiguously diagnosed, with the tumor size ranging from 5 to 30 mm in all transgenic animals analyzed (Fig. 2E). However, in some cases, we observed a large tumor in one lobe, and nodular hyperplasia and/or microcarcinoma in the contralateral lobe. Tumors were divided by fibrous septa in a nested pattern and consisted of a population of round cells with uniform nuclei and amphophilic cytoplasma (Fig. 2F). Finally, the definitive diagnosis of MTC rests on the immunoperoxydase demonstration of CT in malignant cells. Consistent with the histology, CT immunoreactivity was clearly detected in CCH and in MTC of the transgenic mice (Fig. 2 C and G).
Figure 2.
Histology of the thyroid gland in RET-MEN2A transgenic mice. (A) Histologic appearance of a thyroid gland from a nontransgenic mouse. F, follicles; P, parathyroid gland; T, trachea. (Bar = 50 μm.) (B) Distribution of thyroid C cells in a nontransgenic mouse demonstrated by CT immunostaining. Arrows, CT-positive C cells. (Bar = 10 μm.) (C) Diffuse CCH in a transgenic mouse of 4 weeks of age. Note the increased number of C cells throughout the lobe detected by CT immunohistochemistry. (Bar = 50 μm.) (D) Small primary focus of MTC. Malignant cells have infiltrated and obliterated thyroid follicles. (E) MTC with massive invasion of one lobe. Arrows, remnants of thyroid follicles at the peripehry of the tumor mass. (Bar = 50 μm.) (F) Higher magnification of the slide displayed in E showing endocrine lobular and solid pattern characteristic of MTC. (Bar = 20 μm.) (G) Calcitonin immunostaining of MTC cells. Note that a fraction of malignant cells expressed CT, a feature reminiscent of human MTC. (Bar = 10 μm.) (H) Metastatic MTC nodule in a transgenic mouse liver. Large arrow, metastatic nodule; small arrows, normal liver. (Bar = 50 μm.)
Similarly, animals of the 5379 transgenic line developed CCH or MTC with a complete penetrance at 14 months of age. However, four of seven mice from the transgenic line 5322 developed thyroid pathology—i.e., two had a CCH at 6 and 9 months of age, and two had a MTC at 13 months and 16 months of age. Of the three animals that did not present with C cell dysplasia, one was analyzed at 3 weeks, the second one at 9 months, and the latter at 19 months. It is noteworthy that the time lag between CCH and the onset of MTC appears to be longer for mice from the two transgenic lines 5379 and 5322 (on average, CCH at 6 months and MTC at 14 months) compared with mice from the 5319 line (CCH at 3 weeks and MTC at 8 months). Taken together, these results indicate that transgenic mice belonging to the two lines 5319 and 5379 developed MTC with a complete penetrance at 14 months of age, whereas the penetrance of CCH and/or MTC is incomplete for the 5322 transgenic animals.
Organs of all sacrificed transgenic mice were carefully checked and histological analyses were systematically carried out when apparent lesions were detected. Adrenal glands of mice from lines 5319 and 5322 and brains of mice from line 5319 were examined and did not present a pathological aspect at histology (data not shown). Interestingly, among eight different animals whose livers were histologically explored, one transgenic animal from the 5319 line and sacrificed at 15 months of age showed multiple metastatic nodules (Fig. 2H). These nodules expressed the transgene transcript (Fig. 1B). It is worth noting that dissemination of malignant C cells in the liver is a frequent occurrence in advanced stage of human MTC (32).
Abnormal Increase of Plasma CT Correlates with the Presence of Thyroid Pathology.
The recognition of CCH and MTC in predisposed MEN-2 individuals relies on the measurement of serum CT before and after stimulation with pentagastrin or calcium (see ref. 1 and references therein). Abnormal increases in plasma CT after stimulation are a biochemical indication of existing CCH or MTC. To explore whether CCH and MTC were similarly detectable in transgenic mice, we applied the calcium provocative test; the serum CT level was determined by a radioimmunoassay. Transgenic mice and nontransgenic mice of the same sex and age were tested. In nontransgenic mice, the stimulated CT value was 67.80 ± 21 pg/ml, whereas in transgenic mice with CCH the stimulated CT value was 125.8 ± 22 pg/ml and in transgenic mice with MTC, the value was 205.8 ± 15 pg/ml (Table 1). This increase of calcitoninemia was statistically significant (P < 0.001 Fisher–Snedecor test). Taken together, these data demonstrate that elevated levels of CT after calcium stimulation are correlated with the presence of CCH and MTC in transgenic mice.
Table 1.
CT value in calcium provocative test
| Mice | Calcitonin, pg/ml |
|---|---|
| Control (n = 10) | 67.8 ± 21 |
| CT/RET with CCH (n = 15) | 125.8 ± 22 |
| CT/RET with MTC (n = 10) | 205.8 ± 15 |
CT value is expressed as the mean ± SE. The difference between the CT levels in control mice and the two groups of transgenic mice (CT/RET) is statistically significant (P<0.001 Fisher–Snedecor test). The ages of the mice tested are as follows: with CCH, between 2 and 5 months; with MTC, between 8 and 18 months.
Detection of the Aberrant Disulfide-Linked RET Homodimer in MTC of Transgenic Animals.
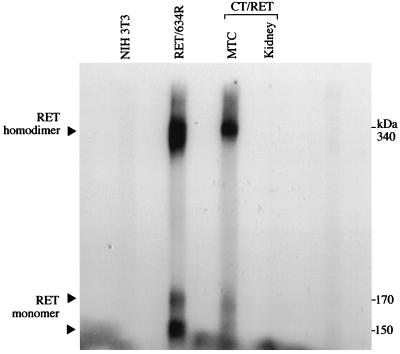
Finally, we examined whether the MEN2A mutant RET protein was expressed in MTC. To discriminate between the endogenous RET protein expressed in murine C cells and the mutant RET protein encoded by the transgene, we exploited the fact that MEN2A and FMTC mutations involving cysteine residues result in the formation of disulfide-linked RET homodimers (25, 26). Using an in vitro kinase assay and nonreducing conditions of SDS/PAGE, three phosphorylated bands at 150, 170, and 340 kDa were detected with lysates of NIH 3T3 cells stably expressing a MEN2A mutant form of RET (Cys-634 → Arg mutant) and with lysates prepared from MTC of transgenic mice (Fig. 3). The 150-kDa and 170-kDa bands, respectively, correspond to an incompletely-glycosylated form of RET and to the fully mature, membrane-bound form of the RET monomer (25). The prominent 340-kDa phosphorylated band was only evidenced under nonreducing conditions, and its size was consistent with the expected size of the covalently linked RET homodimer (25, 26).
Figure 3.
Detection of the RET disulfide-liked homodimer in MTC of transgenic mice (CT/RET). Comparable amounts of proteins were immunoprecipitated with an anti-RET polyclonal serum. The RET protein was then labeled with [γ-32P]ATP in vitro and separated by SDS/PAGE under nonreducing conditions. RET/634R represents NIH 3T3 cells stably expressing the short RET isoform with the Cys-634 → Arg mutation. The 340-kDa phosphorylated protein represents the disulfide-linked RET homodimer. The 170-kDa and the 150-kDa phosphorylated bands correspond, respectively, to the fully mature RET monomer and to an immature, not completely glycosylated form of RET.
DISCUSSION
The development of MTC in MEN-2 patients is viewed as proceeding in several steps (1, 2). The earliest detectable histopathologic feature is a diffuse bilateral CCH which is regarded as a genuine preneoplastic state. With further progression, malignant transformation of these hyperplastic foci results in the development of multicentric and bilateral neoplasms, a clinical criterion that is the hallmark of hereditary MTC (1, 2). The transgenic mice carrying a MEN2A allele of RET fused to the transcriptional regulatory sequences of the CGRP/CT promoter developed CCH and subsequently multifocal and bilateral MTC with a complete penetrance at 14 months of age in two lines (5319, 5379) and with an incomplete penetrance in one line (5322). These tumors were morphologically similar to human MTC. Furthermore, abnormal increases in serum CT levels in response to calcium provocative testing correlated with the occurrence of thyroid pathology in transgenic animals. This finding is reminiscent of the MEN-2 clinical situation in which abnormal calcitoninemia after pentagastrin injection is a sensitive marker of CCH and microscopic MTC (see ref. 1 and references therein). The line-dependent variations of penetrance of thyroid neoplasms and the time lag for tumor formation might be attributable to different levels of transgene expression, an issue we are currently addressing by semiquantitative RT-PCR experiments and protein analysis. Taken together, these results provide evidence that these transgenic mice recapitulate the course of thyroid carcinogenesis that occurs in predisposed MEN-2 individuals.
The transgene mRNA was detected by RT-PCR analyses in adrenal glands of two mouse lines, presumably in the adrenal medulla. However, despite this expression, adrenal glands from these mice did not exhibit histological abnormalities. This observation is surprising since pheochromocytoma occurs in ≈50% of MEN2A gene carriers. One explanation that might account for this lack of concordance between the mouse model and the MEN-2 situation is that the RET-MEN-2A protein is expressed at a level insufficient to exert an effect in adrenal gland. Accordingly, absence of correlation between RT-PCR and protein analyses was noted when kidneys of transgenic animals were examined. The transgene transcript was identified by RT-PCR in kidneys of animals of the mouse line 5319, whereas the RET protein was not detected in renal tissue using an in vitro kinase assay. Alternatively, it is conceivable that the genetic background might influence the penetrance of pheochromocytoma as already described for the c-mos transgenic mice (33).
Our working model postulates that CCH is caused by the constitutively active disulfide-linked RET mutant homodimer that transmits a protracted mitogenic signal to C cells. We do not know at what precise stage of the mouse development the transgene becomes expressed. However, it has been shown that CT can be observed in differentiating C cells from the 14th day of murine embryogenesis when the C cell precursors invade the epithelium of the ultimobranchial pouch (see ref. 34 and references therein). Therefore, it is likely that the covalently linked form of RET we detected in MTC is expressed early during the ontogeny of C cells. In this regard, it is interesting to note that the RET gene appears to be expressed even earlier than the CT gene, since the transcript is detected during the beginning of the multistep migration of the C cell precursors that originate from the postotic rhombencephalic neural crest (35).
The time lag we noticed between CCH and the onset of MTC suggests that additional genetic alterations accumulate, thus conferring clonal advantage and tumorigenic properties to one or a few preneoplastic C cells. Frequent loss of alleles on chromosome arms 1p, 22q, and 3q have been characterized in human MTC (36). Furthermore, a somatic missense mutation at codon 918 (M918T) has been detected in 3 out of 15 MTC that arose in MEN- 2A/FMTC patients carrying a germ-line mutation at one of the five cysteine codons (37). The accumulation of these genetic events are believed to contribute to the emergence of malignant C cells and to promote the tumor progression. In this line of investigation, we are currently studying whether recurrent chromosomal abnormalities are present in MTC of transgenic mice.
Three animal models of MTC have been reported in the literature. In one transgenic strain, expression of the SV40 Large T gene driven from the CGRP/CT promoter sufficed to transform C cells (30). This interesting result was not unexpected since the SV40 large T protein is a potent oncogene capable of transforming cells originating from a wide spectrum of tissues. In the second study, mice carrying a c-mos transgene linked to the mouse sarcoma virus long terminal repeat displayed neurologic lesions, multicentric MTC, and/or pheochromocytomas (33). Consistent with this finding, another study reported that c-mos mRNA is abnormally expressed in human MTC (38). However, no somatic mutations of c-mos have been identified in a series of sporadic MTCs (39). The c-mos gene encodes a cytoplasmic serine/threonine kinase that critically contributes to the control of meiosis and is an upstream activator of mitogen-activating protein kinase (40). Therefore, it is worth considering that the c-mos protein is one of the components of the signal-transduction pathways activated by RET. Future work should elucidate whether c-mos and RET act epistatically. Finally, 70% of the mice heterozygous for a mutation that disrupts the retinoblastoma susceptibility gene (RB) have been shown to develop MTC (41). Interestingly, the human RB1 gene lies on the long arm of chromosome 13 (13q14) and a study reported that the loss of heterozygosity for loci on chromosome arm 13q is present in ≈10% of MTCs (36). However, neither loss nor major rearrangements of the RB1 gene have thus far been detected in MTC (36, 42). Although these results do not preclude that mutations of the RB1 gene are implicated in the tumor progression of MTC, it is also plausible that the high frequency with which MTC arise in RB knock-out animals is specific to mice. Consistent with this latter hypothesis is the fact that mice deficient for RB are not predisposed to retinoblastoma but develop pituitary tumors in almost all cases (41, 43).
To date, establishment of a mouse model of human-inherited cancers has required inactivation of the murine homologs of human tumor-suppressor genes by gene targeting (44). The consequences of MEN2 mutations that convert the RET protooncogene in a dominantly acting transforming gene offered the unique opportunity to create a model of a human hereditary neoplasia using the transgenic technique. In agreement with this notion, the data presented here provide compelling evidence that transgenic mice expressing a MEN2A allele of RET in C cells are predisposed to develop MTC. Overall, these mice should allow several issues to be addressed concerning the role of RET in the pathophysiology of MTC. For instance, it might now be possible to test whether RET activity is responsible for the neuronal properties of malignant C cells (45). Another application would consist of breeding these transgenic mice with mice deficient in genes coding for molecules known to be involved in the RET-mediated signaling pathways and to assess the resulting penetrance of MTC. Such an approach should allow us to determine which signaling proteins contribute to the transforming capacity of RET-MEN-2A in C cells. A similar study has recently permitted the in vivo demonstration that the W/Kit receptor tyrosine kinase is negatively regulated by the tyrosine phosphatase Shp1 (46). Also, the MTC-prone strain of transgenic mice described in this report should constitute a useful animal model for testing drugs, such as protein tyrosine kinase blockers (47), which might inhibit RET function and impede tumor outgrowth. Finally, the contribution of each of RET variants to the transformation process of C cell is as yet unexplored. Our results demonstrate that expression of the short RET isoform activated by a MEN2A mutation promotes C cell tumorigenesis. Future work will unravel the biological properties of the two additional isoforms (RET43 and RET51) harboring the Cys-634 → Arg mutation.
Acknowledgments
We are grateful to M. Fishman for providing us with the CGRP/CT promoter, to D. Goldgar and J. Hall for critical reading of the manuscript, and to E. Schmidt for encouragement during the course of this study. We thank C. Chianale, F. Dessarps-Freichey, and L. Fournier for their valuable technical help; G. Baudry for technical assistance; P. Ardouin for the animal facilities; and P. Auriau for the help with animal care. This work was supported by the Ligue Nationale contre le Cancer, the Ligue Nationale contre le Cancer (Comités Départementaux de l’Ain et de Saône et Loire), the Fondation pour la Recherche Médicale, and the Association de la Recherche contre le Cancer. S.C. and A.P. are recipients of fellowships from the Association de la Recherche contre le Cancer and from the Ligue Nationale contre le Cancer, respectively.
ABBREVIATIONS
- MEN-2
multiple endocrine neoplasia type 2
- MTC
medullary thyroid carcinoma
- FMTC
familial MTC
- CCH
C-cell hyperplasia
- CT
calcitonin
- CGRP
CT gene-related peptide
- SV40
simian virus 40
- RT-PCR
reverse transcription–PCR
References
- 1.DeLellis R A. Lab Invest. 1995;72:494–505. [PubMed] [Google Scholar]
- 2.Goodfellow P J, Wells S A., Jr J Natl Cancer Inst. 1995;87:1515–1523. doi: 10.1093/jnci/87.20.1515. [DOI] [PubMed] [Google Scholar]
- 3.Ishizaka Y, Itoh F, Tahira T, Ikeda I, Sugimura T, Tucker J, Fertitta A, Carrano A V, Nagao M. Oncogene. 1989;4:1519–1521. [PubMed] [Google Scholar]
- 4.Donis-Keller H, Dou S, Chi D, Carlson K M, Toshima K, Lairmore T C, Howe J R, Moley J F, Goodfellow P, Wells S A., Jr Hum Mol Genet. 1993;2:851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- 5.Mulligan L M, Kwok J B J, Healey C S, Elsdon M J, Eng C, Gardner E, Love D R, Mole S E, Moore J K, Papi L, Ponder M A, Telenius H, Tunnacliffe A, Ponder B A J. Nature (London) 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 6.Carlson K M, Dou S, Chi D, Scavarda N, Toshima K, Jackson C E, Wells S A, Jr, Goodfellow P J, Donis-Keller H. Proc Natl Acad Sci USA. 1994;91:1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eng C, Smith D P, Mulligan L M, Nagai M A, Healey C S, Ponder M A, Gardner E, Scheumann G F W, Jackson C E, Tunnacliffe A, Ponder B A J. Hum Mol Genet. 1994;3:237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- 8.Hofstra R M W, Landsvater R M, Ceccherini I, Stulp R P, Stelwagen T, Luo Y, Pasini B, Höppener J W M, Ploos van Amstel H K, Romeo G, Lips C J M, Buys C H C M. Nature (London) 1994;367:375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- 9.Rossel M, Schuffenecker I, Schlumberger M, Bonnardel C, Modigliani E, Gardet P, Navarro J, Luo Y, Romeo G, Lenoir G, Billaud M. Hum Genet. 1995;95:403–406. doi: 10.1007/BF00208964. [DOI] [PubMed] [Google Scholar]
- 10.Tahira T, Ishizaka Y, Itoh F, Sugimura T, Nagao M. Oncogene. 1990;5:97–102. [PubMed] [Google Scholar]
- 11.Myers S M, Eng C, Ponder B A J, Mulligan L M. Oncogene. 1995;11:2039–2045. [PubMed] [Google Scholar]
- 12.Schuchardt A, D’Agati V, Larsson-Bomberg L, Costantini F, Pachnis V. Nature (London) 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 13.Trupp M, Arenas E, Fainzilber M, Nilsson A-S, Sieber B-A, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumäe U, Sariola H, Saarma M, Ibanez C F. Nature (London) 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- 14.Durbec P, Marcos-Gutierrez C V, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, Sariola H, Pachnis V. Nature (London) 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- 15.Treanor J J S, Goodman L, De Sauvage F, Stone D M, Poulsen K T, et al. Nature (London) 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 16.Jing S, Wen D, Yu Y, Holst P L, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis J-C, Hu S, Altrock B W, Fox G M. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez M P, Silos-Santiago I, Frisén J, He B, Lira S A, Barbacid M. Nature (London) 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 18.Pichel J G, Shen L, Sheng H Z, Granholm A C, Drago J, Grinberg A, Lee E J, Huang S P, Saarma M, Hoffer B J, Sariola H, Westphal H. Nature (London) 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 19.Moore M W, Klein R D, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt L F, Ryan A M, Carver-Moore K, Rosenthal A. Nature (London) 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 20.Mulligan L M, Eng C, Healey C S, Clayton D, Kwok J B J, Gardner E, Ponder M A, Frilling A, Jackson C E, Lehnert H, Neumann H P H, Thibodeau S N, Ponder B A J. Nat Genet. 1994;6:70–74. doi: 10.1038/ng0194-70. [DOI] [PubMed] [Google Scholar]
- 21.Schuffenecker I, Billaud M, Calender A, Chambe B, Ginet N, Calmettes C, Modigliani E, Lenoir G M Groupe d’Etude els Tumeurs à Calcitonine. Hum Mol Genet. 1994;3:1939–1943. doi: 10.1093/hmg/3.11.1939. [DOI] [PubMed] [Google Scholar]
- 22.Eng C, Smith D P, Mulligan L M, Healey C S, Zvelebil M J, Stonehouse T J, Ponder M A, Jackson C E, Waterfield M D, Ponder B A J. Oncogene. 1995;10:509–513. [PubMed] [Google Scholar]
- 23.Bolino A, Schuffenecker I, Luo Y, Seri M, Silengo M, Tocco M, Chabrier G, Houdent C, Murat A, Schlumberger M, Tourniaire J, Lenoir G M, Romeo G. Oncogene. 1995;10:2415–2419. [PubMed] [Google Scholar]
- 24.Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, et al. J Am Med Assoc. 1996;276:1575–1579. [PubMed] [Google Scholar]
- 25.Asai N, Iwashita T, Matsuyama M, Takahashi M. Mol Cell Biol. 1995;15:1613–1619. doi: 10.1128/mcb.15.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santoro M, Carlomagno F, Romano A, Bottaro D P, Dathan N A, Grieco M, Fusco A, Vecchio G, Matoskova B, Kraus M H, Di Fiore P P. Science. 1995;267:381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- 27.Borrello M G, Smith D P, Pasini B, Bongarzone I, Greco A, Lorenzo M J, Arighi E, Miranda C, Eng C, Alberti L, Bocciardi R, Mondellini P, Scopsi L, Romeo G, Ponder B A J, Pierotti M A. Oncogene. 1995;11:2419–2427. [PubMed] [Google Scholar]
- 28.Rossel M, Pasini A, Chappuis S, Geneste O, Fournier L, Schuffenecker I, Takahashi M, van Grunsven L A, Urdiales J L, Rudkin B B R, Lenoir G M, Billaud M. Oncogene. 1997;14:265–275. doi: 10.1038/sj.onc.1200831. [DOI] [PubMed] [Google Scholar]
- 29.Songyang Z, Carraway K L, III, Eck M J, Harrison S C, Feldman R A, Mohammadi M, Schlessinger J, Hubbard S R, Smith D P, Eng C, Lorenzo M J, Ponder B A J, Mayer B J, Cantley L C. Nature (London) 1995;373:536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- 30.Baetscher M, Schmidt E, Shimizu A, Leder P, Fishman M C. Oncogene. 1991;6:1133–1138. [PubMed] [Google Scholar]
- 31.Khattab M, Pidoux E, Volle G E, Bouizar Z, Calmettes C, Milhaud G, Moukhtar M S, Treilhou-Lahille F. Bone Miner. 1989;6:249–260. doi: 10.1016/0169-6009(89)90032-9. [DOI] [PubMed] [Google Scholar]
- 32.Ball D W, Baylin B B, de Bustros A C. In: Medullary Thyroid Carcinoma. Braverman L E, Utiger R D, editors. Philadelphia: Lippincott Raven; 1996. pp. 946–960. [Google Scholar]
- 33.Schulz R, Propst F, Rosenberg M P, Linnoila R I, Paules R S, Kovatch R, Ogiso Y, Vande Woude G. Cancer Res. 1992;52:450–455. [PubMed] [Google Scholar]
- 34.Fontaine J. Gen Comp Endocrinol. 1979;37:81–92. doi: 10.1016/0016-6480(79)90049-2. [DOI] [PubMed] [Google Scholar]
- 35.Pachnis V, Mankoo B, Costantini F. Development (Cambridge, UK) 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- 36.Mulligan L M, Gardner E, Smith B A, Mathew C G P, Ponder B A J. Genes Chromosomes Cancer. 1993;6:166–177. doi: 10.1002/gcc.2870060307. [DOI] [PubMed] [Google Scholar]
- 37.Marsh D J, Andrew S D, Eng C, Learoyd D L, Capes A G, Pojer R, Richardson A-L, Houghton C, Mulligan L M, Ponder B A J, Robinson B G. Cancer Res. 1996;56:1241–1243. [PubMed] [Google Scholar]
- 38.Parkar M H, Seid J M, Stringer B M, Ingemansson S, Woodhouse N, Goyns M H. Cancer Lett. 1988;43:185–189. doi: 10.1016/0304-3835(88)90169-3. [DOI] [PubMed] [Google Scholar]
- 39.Eng C, Foster K A, Healey C S, Houghton C, Gayther S A, Mulligan L M, Ponder B A. Br J Cancer. 1996;74:339–341. doi: 10.1038/bjc.1996.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posada J, Yew N, Ahn N G, Vande Woude G F, Cooper J A. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams B O, Remington L, Albert D M, Mukai S, Bronson R T, Jacks T. Nat Genet. 1994;7:480–484. doi: 10.1038/ng0894-480. [DOI] [PubMed] [Google Scholar]
- 42.Holm R, Nesland J M. J Pathol. 1994;172:267–272. doi: 10.1002/path.1711720307. [DOI] [PubMed] [Google Scholar]
- 43.Hu N, Gutsmann A, Herbert D C, Bradley A, Lee W H, Lee E Y. Oncogene. 1994;9:1021–1027. [PubMed] [Google Scholar]
- 44.Williams B O, Jacks T. Curr Opin Genet Dev. 1996;6:65–70. doi: 10.1016/s0959-437x(96)90012-x. [DOI] [PubMed] [Google Scholar]
- 45.Russo A F, Lanigan T M. In: Genetic Mechanisms in Multiple Endocrine Neoplasia Type 2. Nelkin B D, editor. Austin, TX: Landes; 1996. pp. 137–161. [Google Scholar]
- 46.Paulson R F, Vesely S, Siminovitch K A, Bernstein A. Nat Genet. 1996;13:309–315. doi: 10.1038/ng0796-309. [DOI] [PubMed] [Google Scholar]
- 47.Levitzki A, Gazit A. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]