Abstract
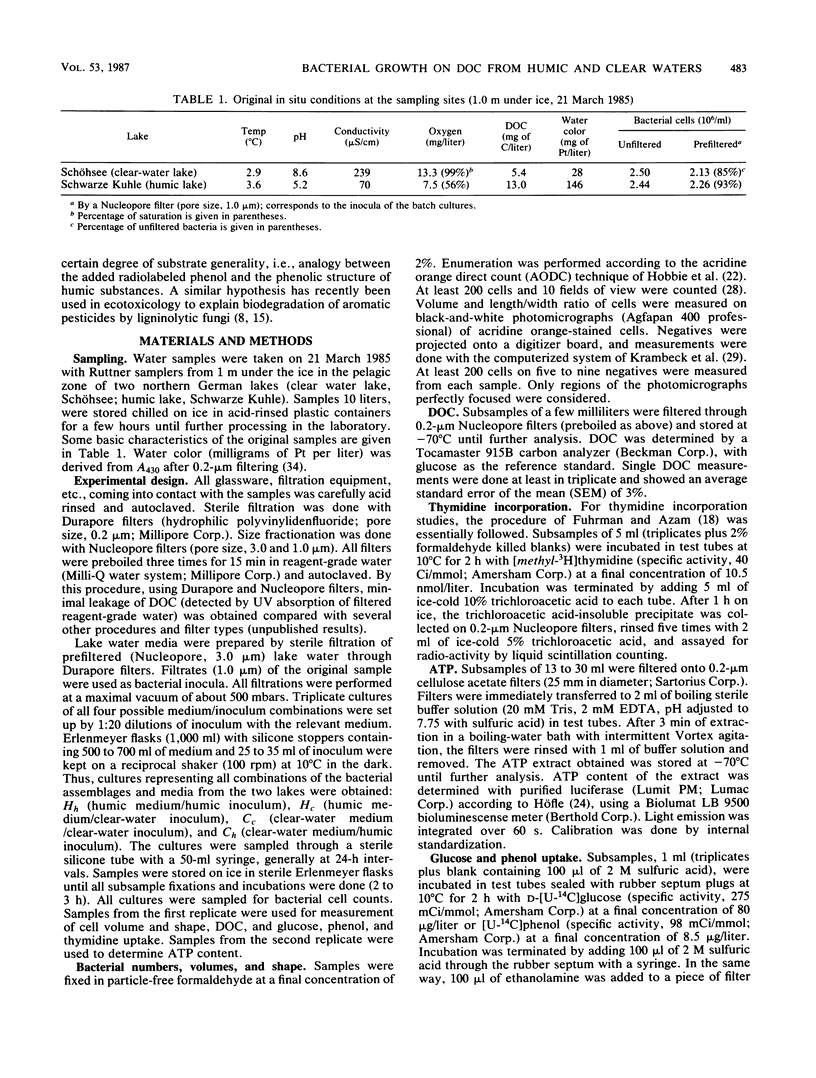
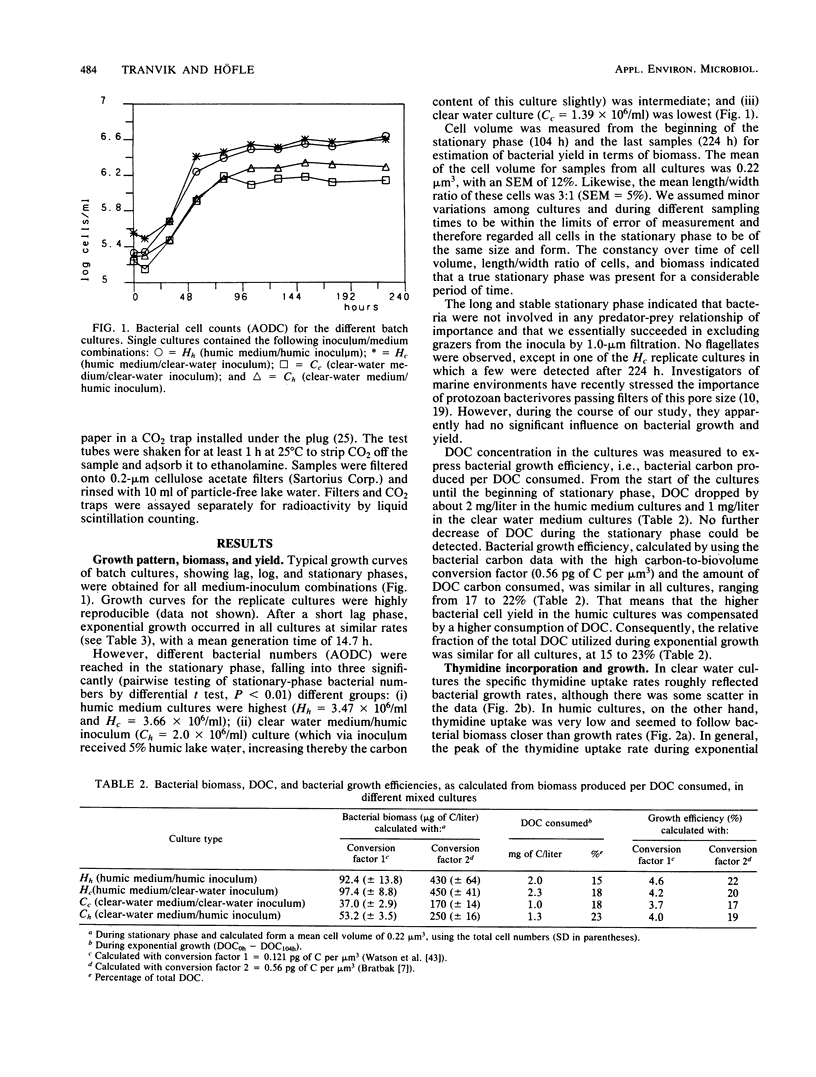
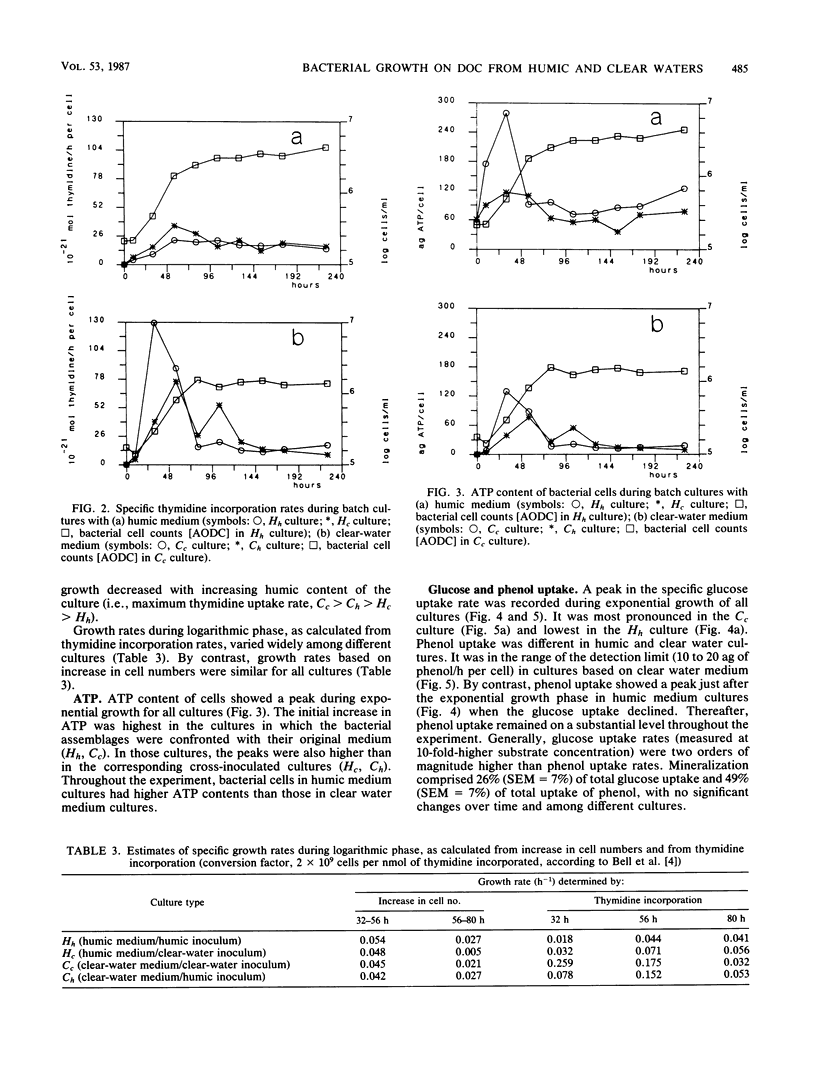
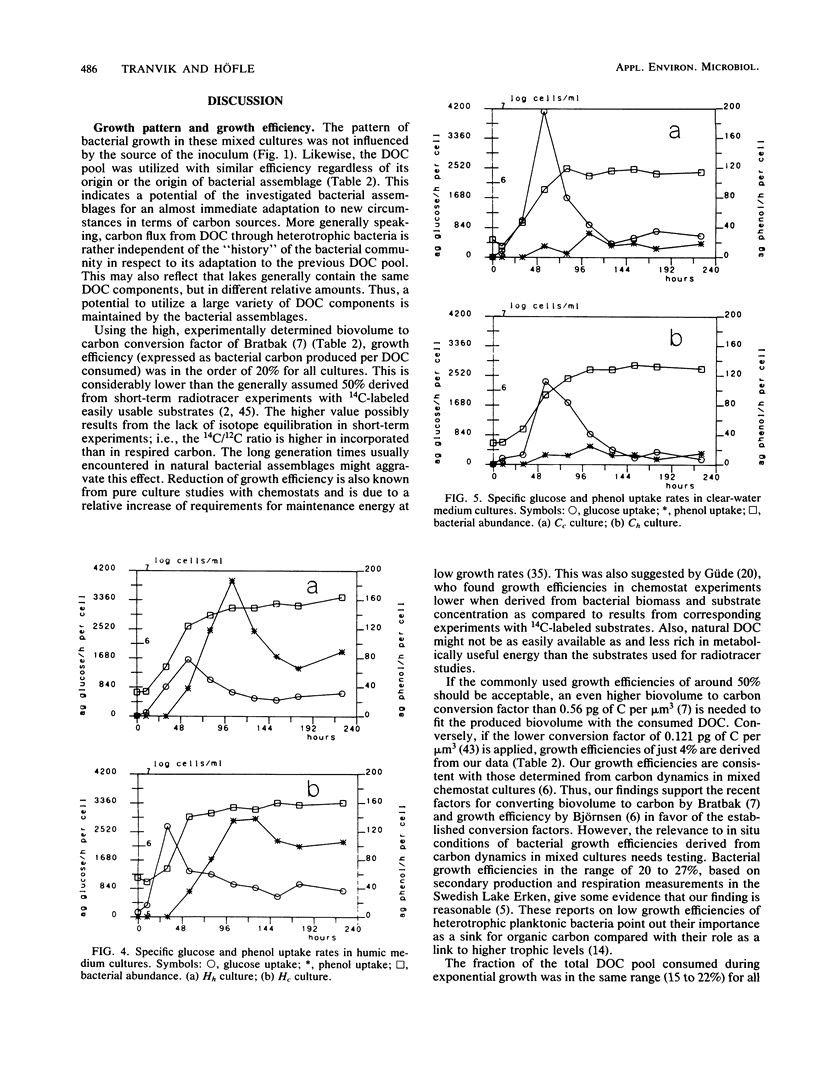
Interactions between bacterial assemblages and dissolved organic carbon (DOC) from different sources were investigated. Mixed batch cultures were set up with water from a humic and a clear-water lake by a 1:20 dilution of the bacterial assemblage (1.0 μm of prefiltered lake water) with natural medium (sterile filtered lake water) in all four possible combinations of the two waters and their bacterial assemblages. Bacterial numbers and biomass, DOC, thymidine incorporation, ATP, and uptake of glucose and phenol were followed in these cultures. Growth curves and exponential growth rates were similar in all cultures, regardless of inoculum or medium. However, bacterial biomass produced was double in cultures based on water from the humic lake. The fraction of DOC consumed by heterotrophic bacteria during growth was in the same range, 15 to 22% of the total DOC pool, in all cultures. Bacterial growth efficiency, calculated from bacterial biomass produced and DOC consumed, was in the order of 20%. Glucose uptake reached a peak during exponential growth in all cultures. Phenol uptake was insignificant in the cultures based on the clear-water medium, but occurred in humic medium cultures after exponential growth. The similarity in the carbon budgets of all cultures indicated that the source of the bacterial assemblage did not have a significant effect on the overall carbon flux. However, fluxes of specific organic compounds differed, as reflected by glucose and phenol uptake, depending on the nature of the DOC and the bacterial assemblage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. T., Kuparinen J. Assessing phytoplankton and bacterioplankton production during early spring in lake erken, sweden. Appl Environ Microbiol. 1984 Dec;48(6):1221–1230. doi: 10.1128/aem.48.6.1221-1230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G. Bacterial biovolume and biomass estimations. Appl Environ Microbiol. 1985 Jun;49(6):1488–1493. doi: 10.1128/aem.49.6.1488-1493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Adenine nucleotide concentrations and turnover rates. Their correlation with biological activity in bacteria and yeast. Adv Microb Physiol. 1977;15:253–306. doi: 10.1016/s0065-2911(08)60318-5. [DOI] [PubMed] [Google Scholar]
- Ducklow H. W., Purdie D. A., Williams P. J., Davies J. M. Bacterioplankton: a sink for carbon in a coastal marine plankton community. Science. 1986 May 16;232(4752):865–867. doi: 10.1126/science.232.4752.865. [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., McManus G. B. Do bacteria-sized marine eukaryotes consume significant bacterial production? Science. 1984 Jun 15;224(4654):1257–1260. doi: 10.1126/science.224.4654.1257. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfle M. G. Degradation of putrescine and cadaverine in seawater cultures by marine bacteria. Appl Environ Microbiol. 1984 Apr;47(4):843–849. doi: 10.1128/aem.47.4.843-849.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfle M. G. Long-Term Changes in Chemostat Cultures of Cytophaga johnsonae. Appl Environ Microbiol. 1983 Nov;46(5):1045–1053. doi: 10.1128/aem.46.5.1045-1053.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Sigda J., Kapuscinski R., Mitchell R. Statistical analysis of the direct count method for enumerating bacteria. Appl Environ Microbiol. 1982 Aug;44(2):376–382. doi: 10.1128/aem.44.2.376-382.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krambeck C., Krambeck H. J., Overbeck J. Microcomputer-assisted biomass determination of plankton bacteria on scanning electron micrographs. Appl Environ Microbiol. 1981 Jul;42(1):142–149. doi: 10.1128/aem.42.1.142-149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R., Pfaender F. K. Influence of naturally occurring humic acids on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol. 1985 Feb;49(2):402–407. doi: 10.1128/aem.49.2.402-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


