Abstract
Recent epidemiologic studies have shown a 40–50% reduction in mortality from colorectal cancer in individuals who take nonsteroidal antiinflammatory drugs on a regular basis compared with those not taking these agents. One property shared by all of these drugs is their ability to inhibit cyclooxygenase (COX), a key enzyme in the conversion of arachidonic acid to prostaglandins. Two isoforms of COX have been characterized, COX-1 and COX-2. COX-2 is expressed at high levels in intestinal tumors in humans and rodents. Human colon cancer cells (Caco-2) were permanently transfected with a COX-2 expression vector or the identical vector lacking the COX-2 insert. The Caco-2 cells, which constitutively expressed COX-2, acquired increased invasiveness compared with the parental Caco-2 cells or the vector transfected control cells. Biochemical changes associated with this phenotypic change included activation of metalloproteinase-2 and increased RNA levels for the membrane-type metalloproteinase. Increased invasiveness and prostaglandin production were reversed by treatment with sulindac sulfide, a known COX inhibitor. These studies demonstrate that constitutive expression of COX-2 can lead to phenotypic changes that alter the metastatic potential of colorectal cancer cells.
Keywords: invasion, metalloproteinase/prostaglandins/sulindac sulfide
Colorectal cancer is the second leading cause of death from cancer in the United States. Even though this disease is curable in early stages, frequently the tumor becomes metastatic by the time an individual presents to their physician with symptoms and thus, the mortality is very high. Therefore, increasing efforts are being focused on developing more effective screening and prevention measures for colorectal cancer. Several studies have reported a 40–50% decrease in mortality from colorectal cancer in persons who are continuous users of aspirin and other nonsteroidal antiinflammatory drugs (NSAIDs) (1–6), suggesting that these drugs may provide a chemoprotective effect. Other studies have shown that the NSAID sulindac is effective in causing regression of adenoma size and number in patients with familial adenomatous polyposis (7–10). Most NSAIDs currently in use inhibit both cyclooxygenase (COX)-1 and COX-2 at their recommended dosages (11–14). Two COX isoforms have been characterized, COX-1 and COX-2. COX-1 is expressed in normal intestine, but its levels do not change in intestinal tumors; however, COX-2 is undetectable in normal intestine (15) and its levels are elevated in up to 85% of colorectal adenocarcinomas (16–18). We have reported that COX-2 mRNA and protein levels are elevated in colonic tumors that develop in rodents following carcinogen treatment (19) and in intestinal adenomas taken from Min mice (20). To examine the role of COX-2 in intestinal epithelial tumorigenesis we have shown that constitutive COX-2 expression in nontransformed rat intestinal epithelial cells leads to inhibition of programmed cell death (21).
The high mortality associated with colorectal cancer is related to its ability to spread beyond the large intestine and invade distant sites. Thus, tumor cell invasion is an extremely important factor for formation of solid tumors and necessary for their spread to distant organs. Proteolysis of basement membrane is one of the most important steps for invasion. Basement membrane consists of laminin, fibronectin, type IV collagen and proteoglycan. Among these basement membrane components, proteolysis of type IV collagen seems most important for invasion to occur because the network fibers of basement membrane are composed of type IV collagen.
The aim of the present study was to investigate the effect of constitutive COX-2 expression in human colon cancer cells on the invasive potential of these cells. Caco-2 cells normally express barely detectable levels of the COX-2 protein, even following growth stimulation. We found that the Caco-2 cells, programmed to constitutively expressed COX-2, acquired increased invasiveness compared with the parental Caco-2 cells or the vector transfected control cells. Biochemical changes associated with this phenotypic change included activation of membrane metalloproteinase-2 (MMP-2) and increased RNA levels for the membrane-type metalloproteinase-1 (MT-MMP-1). The increased invasiveness and prostaglandin production was reversed by treatment with sulindac sulfide, a known COX inhibitor. Our results indicate a direct link between COX-2 and activation of matrix degrading metalloproteinase enzymes.
MATERIALS AND METHODS
Cells and Transfection Protocol.
A 2.1-kb fragment containing the open reading frame for the rat COX-2 gene was isolated and cloned into the eukaryotic expression vector pCB6. This vector was constructed so that transcription of the cDNA is controlled by the cytomegalovirus promoter. This vector also contains a neomycin resistant gene that allows for selection of transfected cells by addition of G418 to the cell culture medium. Caco-2 cells obtained from the American Type Culture Collection were transfected using lipofectamine (GIBCO/BRL) as described (22) and cultured in DMEM containing 1.5 mg/ml of G418 (Sigma), supplemented with 10% fetal bovine serum (HyClone). Using dot blot analysis, the most highly expressing COX-2 clones were identified from a survey of 150 clones and expanded as described (21). Three independently derived control and COX-2 transfected cell lines were characterized and found to have similar phenotypes.
Prostaglandin E2 Measurements.
PGE2 was measured by ELISA (Cayman Chemical, Ann Arbor, MI). PGE2 is the major metabolite of arachidonic acid metabolism in the control and COX-2-overexpressing Caco-2 cells. These measurements were made in triplicate and repeated in three separate experiments.
Immunoblotting.
Immunoblot analysis of cell protein lysates was performed as described (23). Briefly, the cells were lysed for 30 min in RIPA buffer (1× PBS/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS at 10 mg/ml) then clarified cell lysates (50 μg) were denatured and fractionated by 12.5% SDS/PAGE. The proteins were transferred to nitrocellulose filters after electrophoresis, the filters were probed with anti-human COX-1 and COX-2 antibodies (Santa Cruz Biotechnology), developed by the ECL chemiluminescence system (Amersham) and exposed to Kodak XAR5 film. Quantitation was carried out by video densitometry. The MMP-2 and tissue inhibitors of metalloproteinases (TIMP) antibodies were rabbit polyclonal anti-human MMP-2 and TIMP (Oncogene Science). Both the 68- and 62-kDa MMP-2 proteins were detected in the COX-2-overexpressing Caco-2 cells, while only the 68-kDa MMP-2 inactive precursor was detected in the control Caco-2 cells.
Gelatin Zymography.
The metalloproteinases are capable of degrading gelatin; therefore, by incorporating gelatin into the polyacrylamide gel a clear zone indicates the presence of a matrix degrading enzyme. Gelatin zymography was carried out on protein extracts from the Caco-2 cells as described (24–26). The cells were rinsed three times with serum-free Dulbecco’s PBS and cultured with 3 ml of serum-free DMEM for 24 hr. The conditioned medium was collected from subconfluent cultures (90%) of control and COX-expressing Caco-2 cells and separated on 12.5% SDS/PAGE with 1 mg/ml gelatin incorporated into the gel mixture. Following electrophoresis at 4°C, the gel was soaked in 2.5% Triton X-100 to remove the SDS, rinsed in H2O three times, transferred to a bath containing 0.1 M glycine-NaOH (pH 8.3), and incubated at 37°C for 16 hr, then fixed, stained with 0.5% Coomassie blue in 30% isopropanol/10% acetic acid for 1 hr, and destained in 12.5% isopropanol/10% acetic acid.
Northern Blot Analysis.
Total cellular RNA was extracted according to Chirgwin et al. (27). RNA samples (20 μg per lane) were separated on formaldehyde-agarose gels and blotted onto nitrocellulose filters. The blots were hybridized with cDNA probes labeled with [α-32P]dCTP by random primer extension. The COX-2 hybridizations were carried out as described (21). The MT-MMP-1 human cDNA was a gift (H. Sato, Kanazawa University, Japan). After hybridization and washes, the blots were then exposed to x-ray film for autoradiography as described (23). 18S rRNA signals were used as internal control to determine of integrity of RNA and equality of loading among lanes.
Colony Morphology.
To evaluate the morphology of colonies formed in the reconstituted basement membrane, 2 × 103 cells were suspended in 0.5 ml of 1:2 diluted Matrigel (Collaborative Biomedical Products, Bedford, MA). The mixture was then plated into 24-well plates and the cells were grown at 37°C for 2 weeks prior to evaluation and photography.
Invasion Assay.
To quantify invasion, a membrane invasion culture system with 13-mm-diameter filters was used as described (28–30). The upper surface of the polycarbonate filters with 8 micrometer pores was coated with Matrigel (Collaborative Biomedical Products) and placed between the upper and lower well plates of the membrane invasion system. Control Caco-2 or COX-2-expressing Caco-2 cells were cultured in DMEM with 10% fetal bovine serum. In separate experiments, COX-2-expressing Caco-2 cells were cultured in the presence of various concentrations of sulindac sulfide (indicated in Fig. 5) for 24 hr before harvest. After the cells reached confluence they were harvested by mild trypsinization and suspended in DMEM containing 0.1% (wt/vol) BSA.
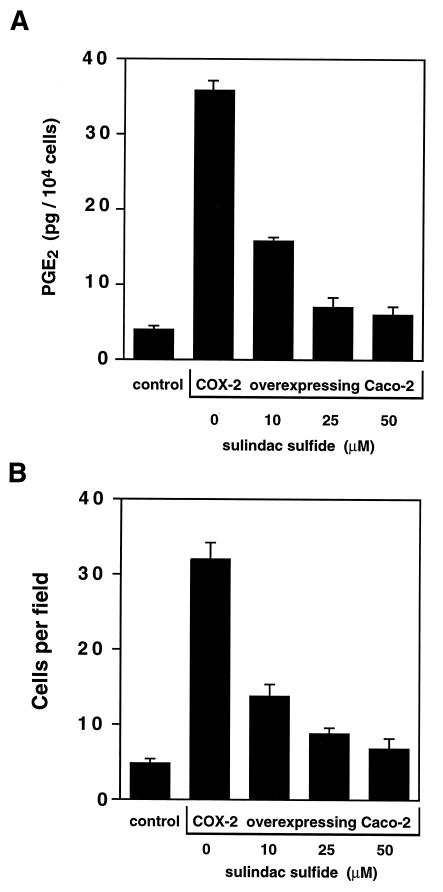
Figure 5.
Dose-dependent sulindac sulfide inhibition of PGE2 production and cell invasion. Either control or COX-2-expressing Caco-2 cells were treated with sulindac sulfide at the indicated concentrations for 24 hr prior to harvest. In A, PGE2 concentrations were determined and the results plotted on the y axis. The cells were then evaluated in the invasion chamber assay described. The number of cells that invaded through the Matrigel and lodged onto the filter were counted and the results plotted on the y axis in B. Results are expressed as number of cells per high power field (×400). Five separate fields were counted per filter and the experiment was repeated four separate times.
Serum-free mouse 3T3 fibroblast conditioned medium was obtained by incubation of these cells for 24 hr. This medium was used as a source of chemoattractant in the lower chamber. Cells (5 × 104) were resuspended in DMEM containing 0.1% BSA and seeded into the upper well of the chamber. Following a 24-hr incubation at 37°C, the cells in the upper chamber and on the upper surface of the Matrigel were mechanically removed, the filters fixed with methanol, stained with hematoxylin/eosin, and the cells on the lower surface were counted manually. The same person counted the cells on all of the filters in a blinded fashion. Five fields were counted per each filter, and the experiment was repeated four separate times.
RESULTS
COX-2 Expression and Prostaglandin Production.
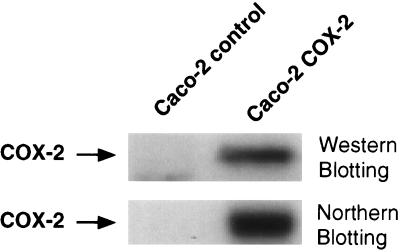
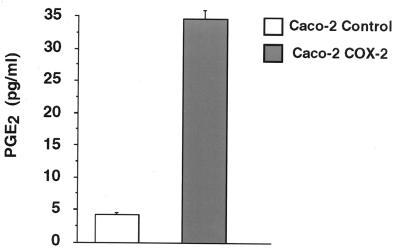
The COX-2 transfected Caco-2 cells exhibited a 10- to 12-fold increase in COX-2 mRNA and protein levels as seen in Fig. 1. COX-1 expression was barely detectable in both the control and COX-2 transfected Caco-2 cell lines (data not shown). This increase in COX-2 expression was accompanied by an increase in prostaglandin synthesis. As shown in Fig. 2, there was an 8-fold increase in PGE2 synthesis when comparing the control Caco-2 cells to the COX-2-overexpressing Caco-2 cells. These results demonstrate that we had selected for a Caco-2 cell line which constitutively expressed COX-2 and that increased COX-2 levels led to increased prostaglandin production. Three independently derived control and COX-2-expressing Caco-2 cell lines were found to have similar characteristics.
Figure 1.
COX-2 expression levels in permanently transfected Caco-2 cells. (Upper) Immunoblotting results from protein extracts (50 μg) taken from control and COX-2-expressing Caco-2 cells. (Lower) Northern blotting results from RNA samples (20 μg total RNA) taken from both control and COX-2-expressing Caco-2 cells. The agarose gel from which this Northern transfer was stained with ethidium bromide to ensure equal loading, and the filter was also probed with an 18 S RNA probe to verify equal loading (data not shown).
Figure 2.
PGE2 production from control and COX-2-expressing Caco-2 cells. Medium was taken from either the control or COX-2-expressing Caco-2 cells and used for ELISA assay for PGE2 levels. Samples were measured in triplicate, and this experiment was repeated three separate times.
Metalloproteinase Activation in COX-2-Expressing Cells.
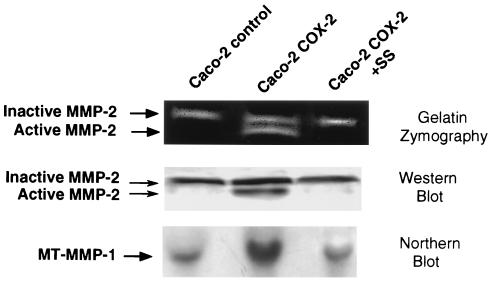
After the COX-2-overexpressing Caco-2 cells were initially isolated we noted that they were difficult to culture on plates precoated with extracellular matrix components, like Matrigel. From simple observation it appeared that these cells were capable of completely degrading the matrix coated onto the culture plates. To further investigate this observation we evaluated the activation state of MMP-2 in the COX-2-overexpressing Caco-2 cells. By employing gelatin zymography we discovered that there were two clear bands in the COX-2-overexpressing cells, one which migrated at 68 kDa and the other at 62 kDa, while we observed only the 68-kDa band in the control Caco-2 cells (Fig. 3 Top). Importantly, treatment of the cells with sulindac sulfide (shown in the third lane) inhibited activation to the 62-kDa band. To confirm that these bands represented MMP-2 we carried out immunoblot analysis of protein extracts from these cells and found the 68- and 62-kDa bands were recognized by monospecific MMP-2 antibodies (Fig. 3 Middle) and that processing to the 62-kDa band was inhibited by treatment of the cells with sulindac sulfide.
Figure 3.
Matrix MMP-2 and MT-MMP-1 expression in Caco-2 cells. (Top) Gelatin zymography of proteins secreted into the cell culture medium. Lane 3 demonstrates the results following treatment with sulindac sulfide (SS; 25 μM) for 24 hr. A 68- and 62-kDa band are seen in the COX-2-expressing Caco-2 cells; whereas, only the inactive 68-kDa band is seen in the control Caco-2 cells. (Middle) Immunoblotting results with an anti-MMP-2 antibody in an identical experiment to that shown in the Top panel. This result confirms the gelatin zymography data shown in the Top panel. (Bottom) Northern blotting results which demonstrates a 2- to 3-fold increase in MT-MMP RNA in the COX-2-expressing Caco-2 cells which is inhibited in lane 3 by treatment of the cells with 25 μM sulindac sulfide for 6 hr. RNA loading controls were carried out as described for the Northern blot in Fig. 1 (data not shown).
The activity of MMP-2 is controlled in at least two ways: proenzyme activation (31, 32) and inhibition of activity by tissue inhibitors of metalloproteinases (TIMP) (33). We investigated the expression levels of TIMP-1 and TIMP-2 in our cell lines by Western blot analysis and found no significant expression of TIMP-1 or TIMP-2 in either cell line (data not shown). To evaluate activation of pro-MMP-2, we examined levels of MT-MMP-1, which is known to cleave the pro-MMP-2 resulting in the active form of the enzyme (34, 35). A cDNA encoding MT-MMP-1 was labeled with a 32P-labeled nucleotide and used in Northern blot analysis to evaluate MT-MMP-1 RNA levels in control Caco-2 and COX-2-expressing Caco-2 cells. We found that MT-MMP-1 RNA levels were increased by 2.8-fold in the COX-2-overexpressing Caco-2 cells (Fig. 3 Bottom). This increase was blocked by treatment of the cells with sulindac sulfide 6 hr prior to isolation of the RNA for Northern analysis (see Fig. 3 Bottom, lane 3). These results indicate that activation of MMP-2 is possibly due to increased MT-MMP-1 expression in COX-2-expressing Caco-2 cells and demonstrate that treatment with sulindac sulfide can block this increase.
COX-2 Expression and Invasiveness of Caco-2 Cells.
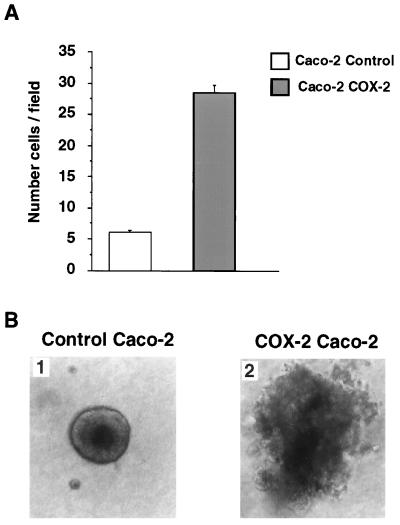
To quantify the invasiveness of the Caco-2 cells we employed a simple invasion chamber assay method (28). To carry out these experiments an invasion assay was set up in which there was an upper and lower chamber, separated by a filter onto which the cells are trapped and cell number counted. The bottom of the upper chamber was coated with Matrigel and filled with DMEM. The lower chamber was filled with serum-free conditioned 3T3 fibroblast medium to act as a chemoattractant. Control Caco-2 or COX-2-overexpressing Caco-2 cells (2 × 104) were loaded into the upper chamber and following a 24-hr incubation period the number of cells attached to the filter below the Matrigel layer was counted. The results of this experiment (shown in Fig. 4A) clearly indicates that about 6 times more of the COX-2-expressing Caco-2 cells were able to invade through the Matrigel and lodge onto the filter. This result indicates the constitutive expression of COX-2 leads to increased invasiveness of human colon cancer cells. To further characterize these cells we examined their ability to form colonies in Matrigel. The results (shown in Fig. 4B) indicate that the morphology of the colonies formed by COX-2-expressing Caco-2 cells were more extensive with a significant amount of lateral invasion into the matrix components leading to the formation of large colonies with irregular borders. Because the control and COX-2-overexpressing Caco-2 cells had identical growth rates (data not shown), their difference in colony morphology may be due to the activation of the matrix degrading metalloproteinase enzyme, MMP-2 in the COX-2-expressing cells. Collectively, these results indicate a marked increase in invasiveness in human colon cancer cells programmed to express the COX-2 gene constitutively. Taking the previous results shown in Fig. 3 into account, this increased invasiveness may be due to an increased activation of MMP-2.
Figure 4.
Invasiveness and colony formation of Caco-2 cells. (A) Results of the invasion chamber assays quantitating the number of cells that migrate through the Matrigel layer to the filter placed on the bottom of the upper chamber. Cells were counted at a magnification of ×400. (B) Morphology of the colonies formed by control and COX-2-expressing Caco-2 cells. Cells were plated in Matrigel and incubated for 2 weeks prior to photography. (B1) Results for the control Caco-2 cells. (B2) Results for the COX-2-expressing Caco-2 cells. (×200.)
Effect of the NSAID Sulindac Sulfide on Prostaglandin Production and Invasiveness.
To determine if the invasive phenotype could be altered by COX inhibitors, we added sulindac sulfide to the cells at various concentrations and then determined their PGE2 levels and invasiveness. The results shown in Fig. 5A indicate that sulindac sulfide inhibited PGE2 production in a dose-dependent fashion with maximal effectiveness above 10 μM in our assay system. The dose response for inhibition of invasiveness, shown if Fig. 5B, has a remarkably similar profile. These results clearly demonstrate that the increased invasiveness caused by COX-2 overexpression could be reversed in a dose-dependent fashion by treatment with the COX inhibitor, sulindac sulfide.
DISCUSSION
A remarkable amount of evidence, collected from multiple different experimental systems, indicates that NSAIDs reduce mortality for colorectal cancer in humans and decrease tumor multiplicity in rodent models of intestinal tumorigenesis. NSAIDs also reduce adenoma size and number in familial adenomatous polyposis patients. One common property shared by most NSAIDs is their ability to inhibit COX enzymes. Two isoforms for COX have been described, COX-1 and COX-2. We and others have shown that COX-2 levels are markedly increased in human colorectal adenocarcinomas and in intestinal tumors that develop in carcinogen-treated rats and Min mice. Does COX-2 play a role in intestinal tumorigenesis?
In an effort to better understand the role of COX-2 in colorectal carcinogenesis, we permanently transfected Caco-2 cells with a COX-2 expression vector and selected for cells that constitutively express the COX-2 gene. We verified that our COX-2-transfected cell lines maintained high levels of COX-2 expression and prostaglandin production. Phenotypically, the COX-2-expressing cells possessed potent extracellular matrix degrading activity that was likely due to activation of the matrix metalloproteinase, MMP-2 (Fig. 3). A plausible reason for the increased activation of MMP-2 was identified in that the MT-MMP-1 mRNA levels were increased in the COX-2-expressing cells. The molecular basis for increased MT-MMP-1 expression is currently under investigation. We have been able to demonstrate that treatment with sulindac sulfide inhibits this activation (see Fig. 3). To quantify the phenotypic changes associated with the COX-2-expressing Caco-2 cells, we evaluated their invasiveness in a chamber assay. We found that the COX-2-expressing cells were 6 times more invasive than the control Caco-2 cells and that they remain invasive when plated in an environment where they were surrounded by Matrigel. They form large irregular colonies compared with the control cells, as depicted in Fig. 4B.
To test the hypothesis that NSAIDs could alter the increased invasive properties of these cells, we treated them with sulindac sulfide and found that their increased invasiveness was almost completely inhibited in a dose-dependent fashion (Fig. 5B). These results indicate that COX-2 activity may be required to maintain the altered phenotype of increased invasiveness. Because we lack knowledge of the mechanisms by which NSAIDs cause their chemoprotective effects, these results are intriguing and may help to explain one aspect of this process. NSAID use in humans has been associated with a 50% decrease in mortality from colorectal cancer. Our results reported here indicate that activation of MMP-2 can be modulated by COX-2 and treatment with a COX inhibitor reverses increased invasiveness of Caco-2 cells and inhibits activation of MMP-2.
Acknowledgments
We would like to thank Drs. H. Sato and M. Seiki for generously providing us with the MT-MMP-1 cDNA. We acknowledge support from the A. B. Hancock, Jr., Memorial Laboratory, the Lucille P. Markey Charitable Trust, the U.S. Public Health Service (Grants DK 47297 to R.N.D. and NIHES 00267 to R.N.D.), and a Veterans Affairs Merit grant (to R.N.D.). R.N.D. is a recipient of a Veterans Affairs Research Associate career development award, Boehringer Ingelheim New Investigator Award, and is an American Gastroenterological Association Gastroenterology Young Investigator Award recipient.
ABBREVIATIONS
- COX
cyclooxygenase
- NSAID
nonsteroidal antiinflammatory drug
- MMP-2
metalloproteinase-2
- MT MMP
membrane-type metalloproteinase
- PGE2
prostaglandin E2
- TIMP
tissue inhibitors of metalloproteinases
References
- 1.Thun M J, Namboodiri M M, Heath C W J. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 2.Thun M J, Namboodiri M M, Calle E E, Flanders W D, Heath C W J. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 3.Marnett L J. Cancer Res. 1992;52:5575–5589. [PubMed] [Google Scholar]
- 4.Marnett L J. Prev Med. 1995;24:103–106. doi: 10.1006/pmed.1995.1017. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Rimm E B, Stampfer M J, Colditz G A, Ascherio A, Willett W C. Ann Intern Med. 1994;121:241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Egan K M, Hunter D J, Stampfer M J, Colditz G A, Willett W C, Speizer F E. N Eng J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 7.Waddell W R, Loughry R W. J Surg Oncol. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 8.Waddell W R, Gasner G F, Cerise E J, Loughry R W. Am J Surg. 1989;157:175–178. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]
- 9.Giardiello F M, Hamilton S R, Krush A J, Piantadosi S, Hylind L M, Celano P, Booker S V, Robinson C R, Offerhaus G J. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 10.Giardiello F M, Offerhaus G J A, DuBois R N. Eur J Cancer. 1995;31A:1071–1076. doi: 10.1016/0959-8049(95)00137-8. [DOI] [PubMed] [Google Scholar]
- 11.Meade E A, Smith W L, DeWitt D L. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 12.Laneuville O, Breuer D K, Dewitt D L, Hla T, Funk C D, Smith W L. J Pharmacol Exp Ther. 1994;271:927–934. [PubMed] [Google Scholar]
- 13.O’Neill G P, Mancini J A, Kargman S, Yergey J, Kwan M Y, Falgueyret J P, Abramovitz M, Kennedy B P, Ouellet M, Cromlish W, Culp S, Evans J F, Ford-Hutchinson A W, Vickers P J. Mol Pharmacol. 1994;45:245–254. [PubMed] [Google Scholar]
- 14.Gierse J K, Hauser S D, Creely D P, Koboldt C, Rangwala S H, Isakson P C, Seibert K. Biochem J. 1995;305:479–484. doi: 10.1042/bj3050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kargman S, Charleson S, Cartwright M, Frank J, Mancini J, Evans J, O’Neill G. Gastroenterology. 1996;111:445–454. doi: 10.1053/gast.1996.v111.pm8690211. [DOI] [PubMed] [Google Scholar]
- 16.Eberhart C E, Coffey R J, Radhika A, Giardiello F M, Ferrenbach S, DuBois R N. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 17.Kargman S, O’Neill G, Vickers P, Evans J, Mancini J, Jothy S. Cancer Res. 1995;55:2556–2559. [PubMed] [Google Scholar]
- 18.Sano H, Kawahito Y, Wilder R L, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 19.DuBois R N, Radhika A, Reddy B S, Entingh A J. Gastroenterology. 1996;110:1259–1262. doi: 10.1053/gast.1996.v110.pm8613017. [DOI] [PubMed] [Google Scholar]
- 20.Williams C W, Luongo C, Radhika A, Zhang T, Lamps L W, Nanney L B, Beauchamp R D, DuBois R N. Gastroenterology. 1996;111:1134–1140. doi: 10.1016/s0016-5085(96)70083-5. [DOI] [PubMed] [Google Scholar]
- 21.Tsujii M, DuBois R N. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 22.DuBois R N, Tsujii M, Bishop P, Awad J A, Makita K, Lanahan A. Am J Physiol. 1994;266:G822–G827. doi: 10.1152/ajpgi.1994.266.5.G822. [DOI] [PubMed] [Google Scholar]
- 23.DuBois R N, Shao J, Sheng H, Tsujii M, Beauchamp R D. Cancer Res. 1996;56:733–737. [PubMed] [Google Scholar]
- 24.Heussen C, Dowdle E B. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 25.Alexander C M, Werb Z. J Cell Biol. 1992;118:727–739. doi: 10.1083/jcb.118.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behrendtsen O, Alexander C M, Werb Z. Development (Cambridge, UK) 1992;114:447–456. doi: 10.1242/dev.114.2.447. [DOI] [PubMed] [Google Scholar]
- 27.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 28.Albini A, Iwamoto Y, Kleinman H K, Martin G R, Aaronson S A, Kozlowski J M, McEwan R N. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 29.Melchiori A, Albini A, Ray J M, Stetler-Stevenson W G. Cancer Res. 1992;52:2353–2356. [PubMed] [Google Scholar]
- 30.Kobayashi H, Ohi H, Sugimura M, Shinohara H, Fujii T, Terao T. Cancer Res. 1992;52:3610–3614. [PubMed] [Google Scholar]
- 31.Cao J, Sato H, Takino T, Seiki M. J Biol Chem. 1995;270:801–805. doi: 10.1074/jbc.270.2.801. [DOI] [PubMed] [Google Scholar]
- 32.Strongin A Y, Collier I, Bannikov G, Marmer B L, Grant G A, Goldberg G I. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 33.Denhardt D T, Feng B, Edwards D R, Cocuzzi E T, Malyankar U M. Pharmacol Ther. 1993;59:329–341. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- 34.Nomura H, Sato H, Seiki M, Mai M, Okada Y. Cancer Res. 1995;55:3263–3266. [PubMed] [Google Scholar]
- 35.Takino T, Sato H, Shinagawa A, Seiki M. J Biol Chem. 1995;270:23013–23020. doi: 10.1074/jbc.270.39.23013. [DOI] [PubMed] [Google Scholar]