Abstract
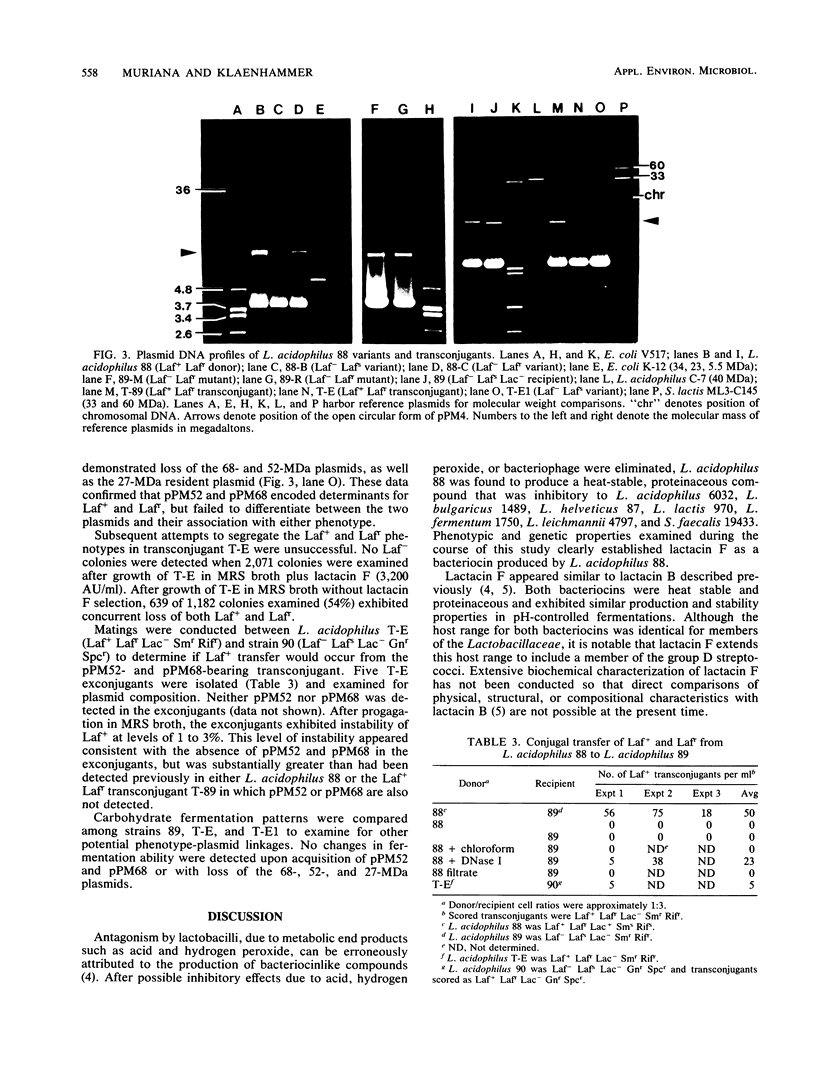
Lactobacillus acidophilus 88 produced a bacteriocin, designated lactacin F, that demonstrated inhibitory activity toward L. acidophilus 6032, L. lactis 970, L. helveticus 87, L. bulgaricus 1489, L. leichmanii 4797, L. fermentum 1750, and Streptococcus faecalis 19433. Production of lactacin F was pH dependent and could be maximized in MRS broth cultures maintained at pH 7.0. Lactacin F was heat stable and sensitive to ficin, proteinase K, trypsin, and Bacillus subtilis protease. L. acidophilus 88 harbored plasmids of 4 and 27 megadaltons. Variants of L. acidophilus 88 which were deficient in lactacin F production (Laf+) and lactacin F immunity (Laft) retained the two resident plasmids. A Laf− Lafs derivative, L. acidophilus 89, was used as a recipient in agar surface mating experiments with L. acidophilus 88 (Laf+ Laft). Two types of Laf+ Laft transconjugants were recovered. One type (T-E) had acquired two plasmids of 68 (pPM68) and 52 (pPM52) megadaltons that were not detected in either the conjugal donor or the other type of Laf+ Laft transconjugants (T-89). Laf+ and Laft were unstable in the plasmid-bearing transconjugant. Plasmid analysis of Laf− Lafs variants revealed that pPM52 and pPM68 were cured with loss of Laf+ and Laft. Bacteriocin production and immunity phenotypes were genetically stable in Laf+ Laft transconjugants not harboring pPM52 and pPM68, suggesting chromosomal integration of the transferred determinants. The data demonstrated intragenic conjugation in L. acidophilus and provided direct evidence for involvement of transient plasmid determinants in Laf+ and Laft.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Genetic and physical characterization of recombinant plasmids associated with cell aggregation and high-frequency conjugal transfer in Streptococcus lactis ML3. J Bacteriol. 1984 Jun;158(3):954–962. doi: 10.1128/jb.158.3.954-962.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero F., Bouanchaud D., Martinez-Perez M. C., Fernandez C. Microcin plasmids: a group of extrachromosomal elements coding for low-molecular-weight antibiotics in Escherichia coli. J Bacteriol. 1978 Aug;135(2):342–347. doi: 10.1128/jb.135.2.342-347.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot S. F., Klaenhammer T. R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol. 1983 Jun;45(6):1808–1815. doi: 10.1128/aem.45.6.1808-1815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot S. F., Klaenhammer T. R. Purification and characterization of the Lactobacillus acidophilus bacteriocin lactacin B. Antimicrob Agents Chemother. 1984 Sep;26(3):328–334. doi: 10.1128/aac.26.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE KLERK H. C., COETZEE J. N. Antibiosis among lactobacilli. Nature. 1961 Oct 28;192:340–341. doi: 10.1038/192340a0. [DOI] [PubMed] [Google Scholar]
- Gibson E. M., Chace N. M., London S. B., London J. Transfer of plasmid-mediated antibiotic resistance from streptococci to lactobacilli. J Bacteriol. 1979 Jan;137(1):614–619. doi: 10.1128/jb.137.1.614-619.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Extrachromosomally determined antibiotic production. Annu Rev Microbiol. 1978;32:373–392. doi: 10.1146/annurev.mi.32.100178.002105. [DOI] [PubMed] [Google Scholar]
- Iwata M., Mada M., Ishiwa H. Protoplast fusion of Lactobacillus fermentum. Appl Environ Microbiol. 1986 Aug;52(2):392–393. doi: 10.1128/aem.52.2.392-393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger M. C., Klaenhammer T. R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J Bacteriol. 1986 Aug;167(2):439–446. doi: 10.1128/jb.167.2.439-446.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Wickner L. J., Chassy B. M. Production and Regeneration of Lactobacillus casei Protoplasts. Appl Environ Microbiol. 1984 Nov;48(5):994–1000. doi: 10.1128/aem.48.5.994-1000.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. H., Savage D. C. Genetic transformation of rifampicin resistance in Lactobacillus acidophilus. J Gen Microbiol. 1986 Aug;132(8):2107–2111. doi: 10.1099/00221287-132-8-2107. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McCormick E. L., Savage D. C. Characterization of Lactobacillus sp. strain 100-37 from the murine gastrointestinal tract: ecology, plasmid content, and antagonistic activity toward Clostridium ramosum H1. Appl Environ Microbiol. 1983 Nov;46(5):1103–1112. doi: 10.1128/aem.46.5.1103-1112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihelc V. A., Duncan C. L., Chambliss G. H. Characterization of a bacteriocinogenic plasmid in Clostridium perfringens CW55. Antimicrob Agents Chemother. 1978 Nov;14(5):771–779. doi: 10.1128/aac.14.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Evidence for Plasmid Linkage of Restriction and Modification in Streptococcus cremoris KH. Appl Environ Microbiol. 1981 Dec;42(6):944–950. doi: 10.1128/aem.42.6.944-950.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetter K. O. Evidence for frequent lysogeny in lactobacilli: temperate bacteriophages within the subgenus Streptobacterium. J Virol. 1977 Nov;24(2):685–689. doi: 10.1128/jvi.24.2.685-689.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti G. C., Hinsdill R. D. Production and mode of action of lactocin 27: bacteriocin from a homofermentative Lactobacillus. Antimicrob Agents Chemother. 1975 Feb;7(2):139–145. doi: 10.1128/aac.7.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovo M., Morelli L., Bottazzi V. Drug resistance plasmids in Lactobacillus acidophilus and Lactobacillus reuteri. Appl Environ Microbiol. 1982 Jan;43(1):50–56. doi: 10.1128/aem.43.1.50-56.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovo M., Morelli L., Bottazzi V., Gasson M. J. Conjugal Transfer of Broad-Host-Range Plasmid pAMbeta1 into Enteric Species of Lactic Acid Bacteria. Appl Environ Microbiol. 1983 Sep;46(3):753–755. doi: 10.1128/aem.46.3.753-755.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Tersch M. A., Carlton B. C. Megacinogenic plasmids of Bacillus megaterium. J Bacteriol. 1983 Aug;155(2):872–877. doi: 10.1128/jb.155.2.872-877.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R., Rowsome W., Tsao J., Visentin L. P. Identification and characterization of Col plasmids from classical colicin E-producing strains. J Bacteriol. 1981 Aug;147(2):569–577. doi: 10.1128/jb.147.2.569-577.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. A., Warner P. J. Plasmid profiles and transfer of plasmid-encoded antibiotic resistance in Lactobacillus plantarum. Appl Environ Microbiol. 1985 Nov;50(5):1319–1321. doi: 10.1128/aem.50.5.1319-1321.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]





