Abstract
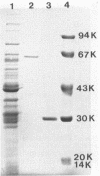
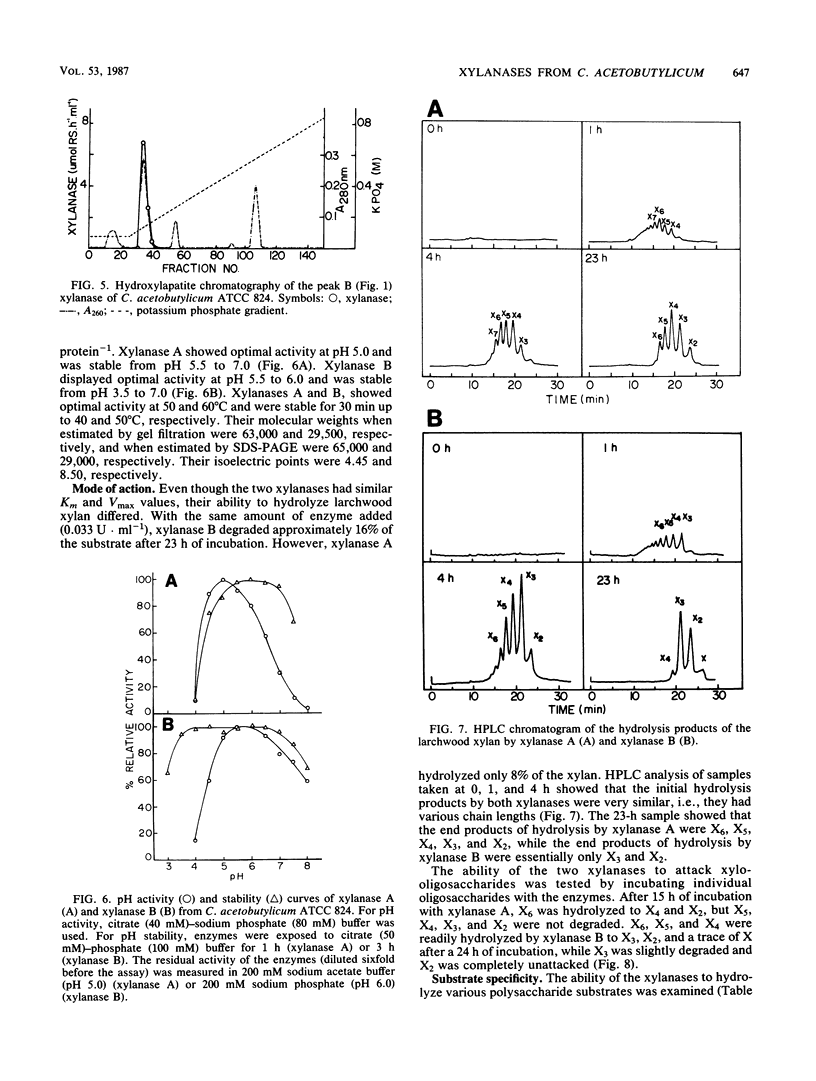
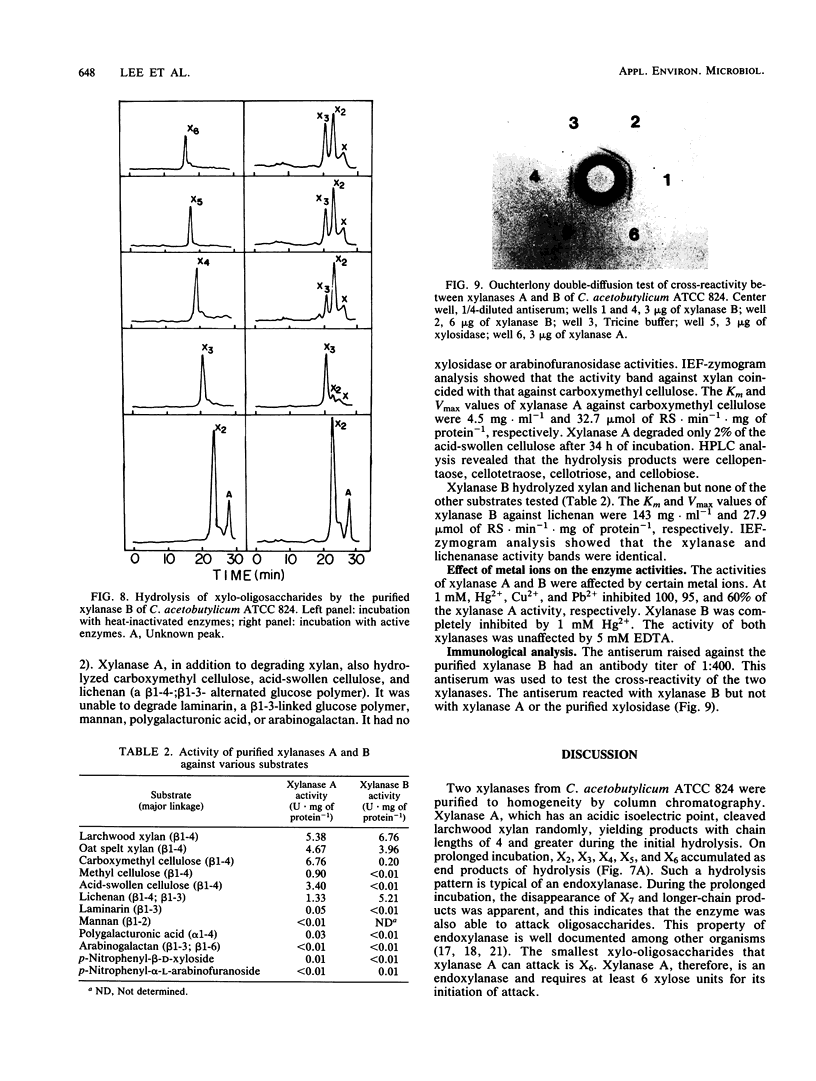
Two endoxylanases produced by C. acetobutylicum ATCC 824 were purified to homogeneity by column chromatography. Xylanase A, which has a molecular weight of 65,000, hydrolyzed larchwood xylan randomly, yielding xylohexaose, xylopentaose, xylotetraose, xylotriose, and xylobiose as end products. Xylanase B, which has a molecular weight of 29,000, also hydrolyzed xylan randomly, giving xylotriose and xylobiose as end products. Xylanase A hydrolyzed carboxymethyl cellulose with a higher specific activity than xylan. It also exhibited high activity on acid-swollen cellulose. Xylanase B showed practically no activity against either cellulose or carboxymethyl cellulose but was able to hydrolyze lichenan with a specific activity similar to that for xylan. Both xylanases had no aryl-β-xylosidase activity. The smallest oligosaccharides degraded by xylanases A and B were xylohexaose and xylotetraose, respectively. The two xylanases demonstrated similar Km and Vmax values but had different pH optima and isoelectric points. Ouchterlony immunodiffusion tests showed that xylanases A and B lacked antigenic similarity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chander S., Rath G. K., Chandra M., Datta N. R. Breast carcinoma in a young girl. Indian J Cancer. 1986 Dec;23(4):217–221. [PubMed] [Google Scholar]
- Dekker R. F., Richards G. N. Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem. 1976;32:277–352. doi: 10.1016/s0065-2318(08)60339-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Gibbins L. N. Cellulolytic Activity of Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Aug;50(2):220–228. doi: 10.1128/aem.50.2.220-228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Gibbins L. N. Xylanolytic Activity of Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Oct;50(4):1068–1076. doi: 10.1128/aem.50.4.1068-1076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W. Isolation and Some Properties of a beta-d-Xylosidase from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1987 Apr;53(4):651–654. doi: 10.1128/aem.53.4.651-654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie C. R., Bilous D., Johnson K. G. Purification and characterization of an exoglucanase from Streptomyces flavogriseus. Can J Microbiol. 1984 Sep;30(9):1171–1178. doi: 10.1139/m84-183. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]