Abstract
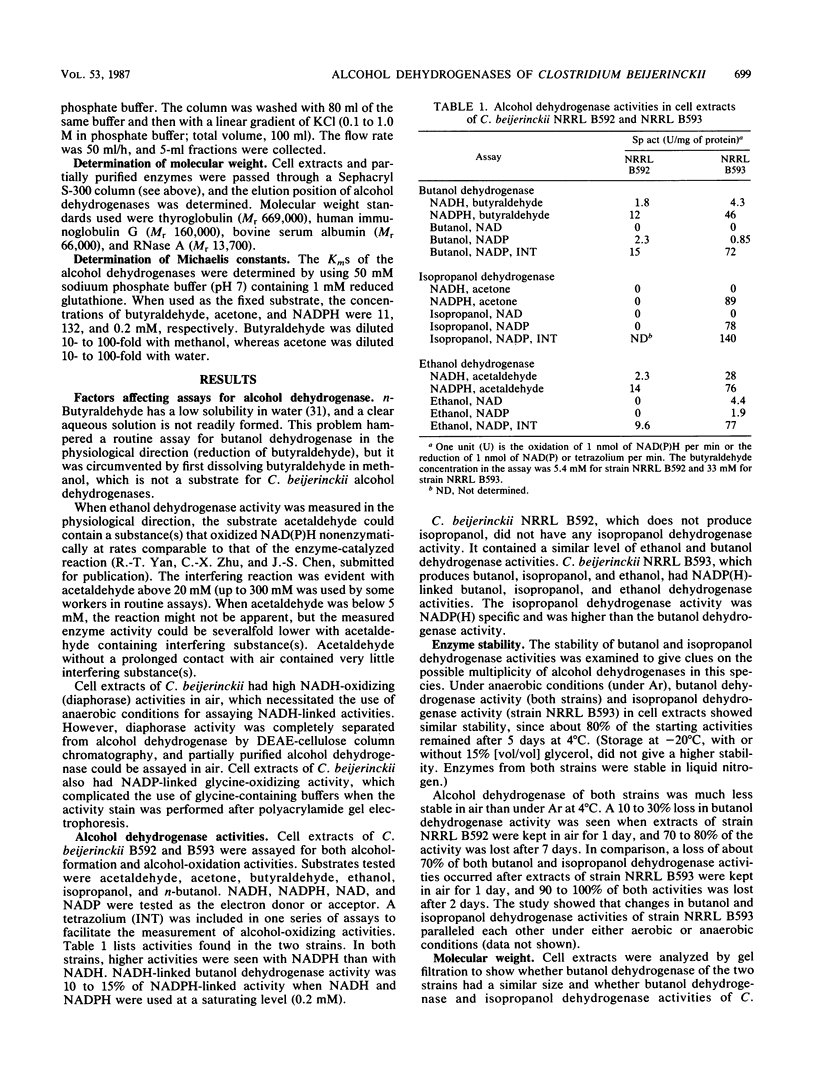
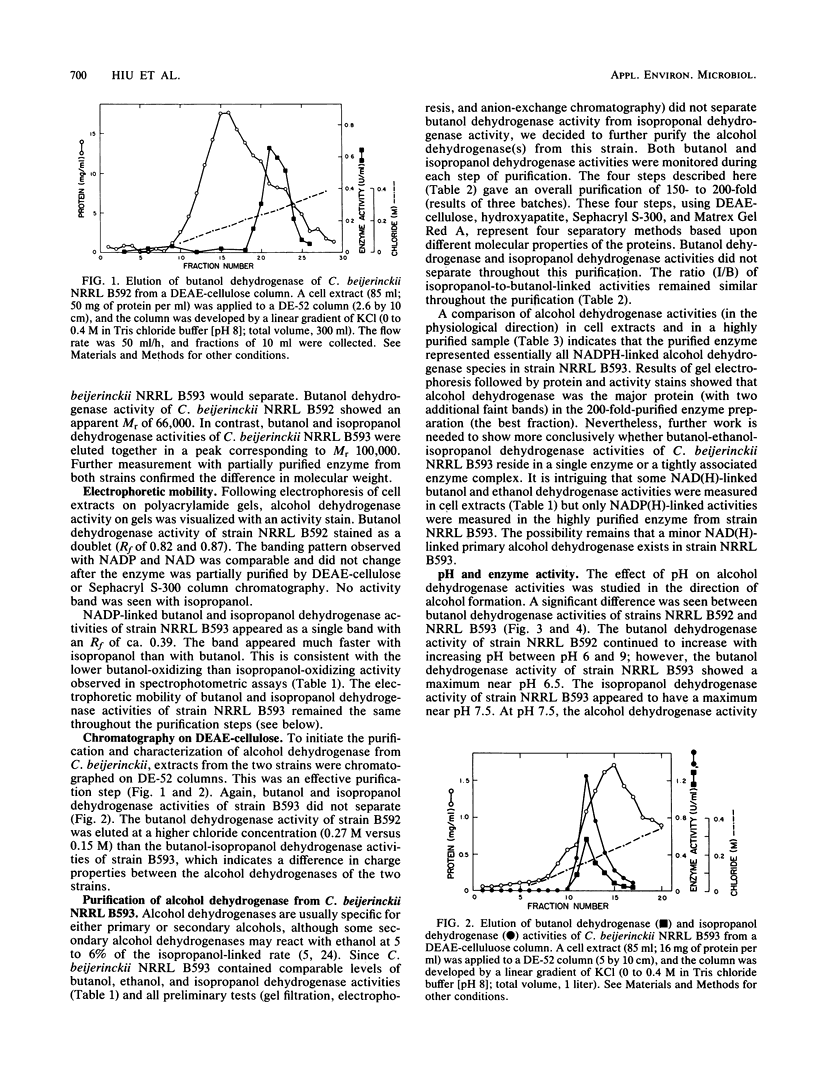
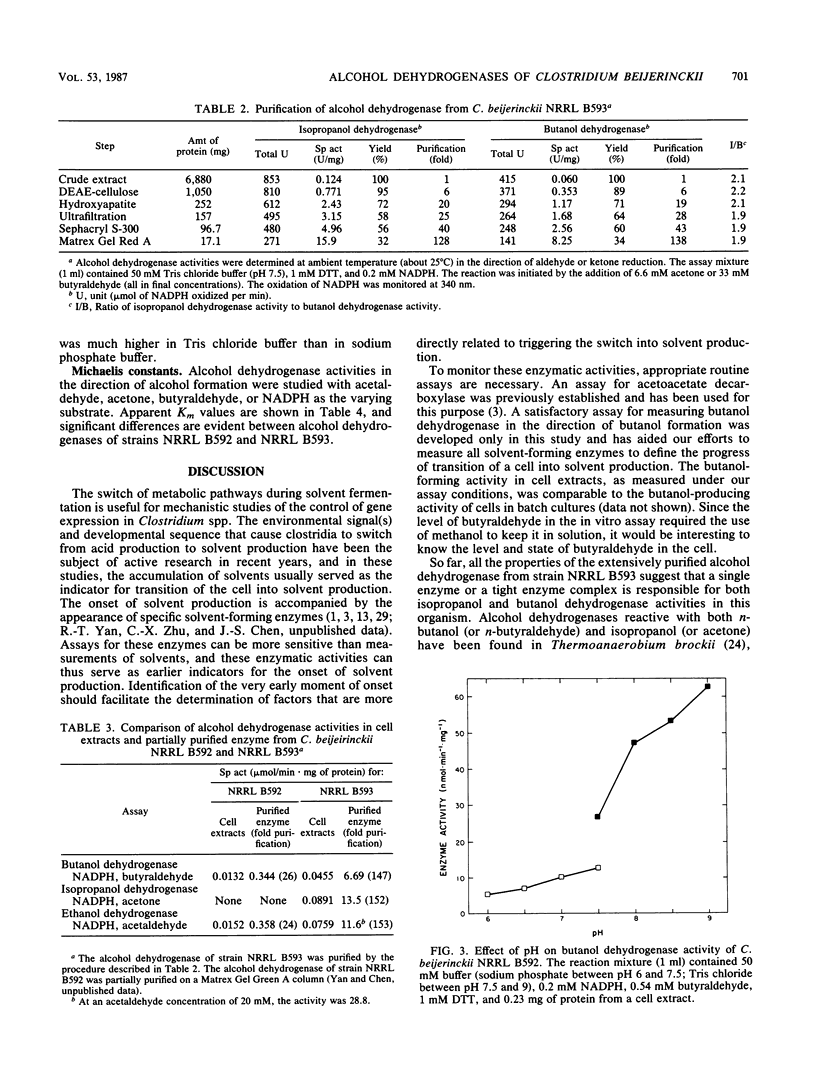
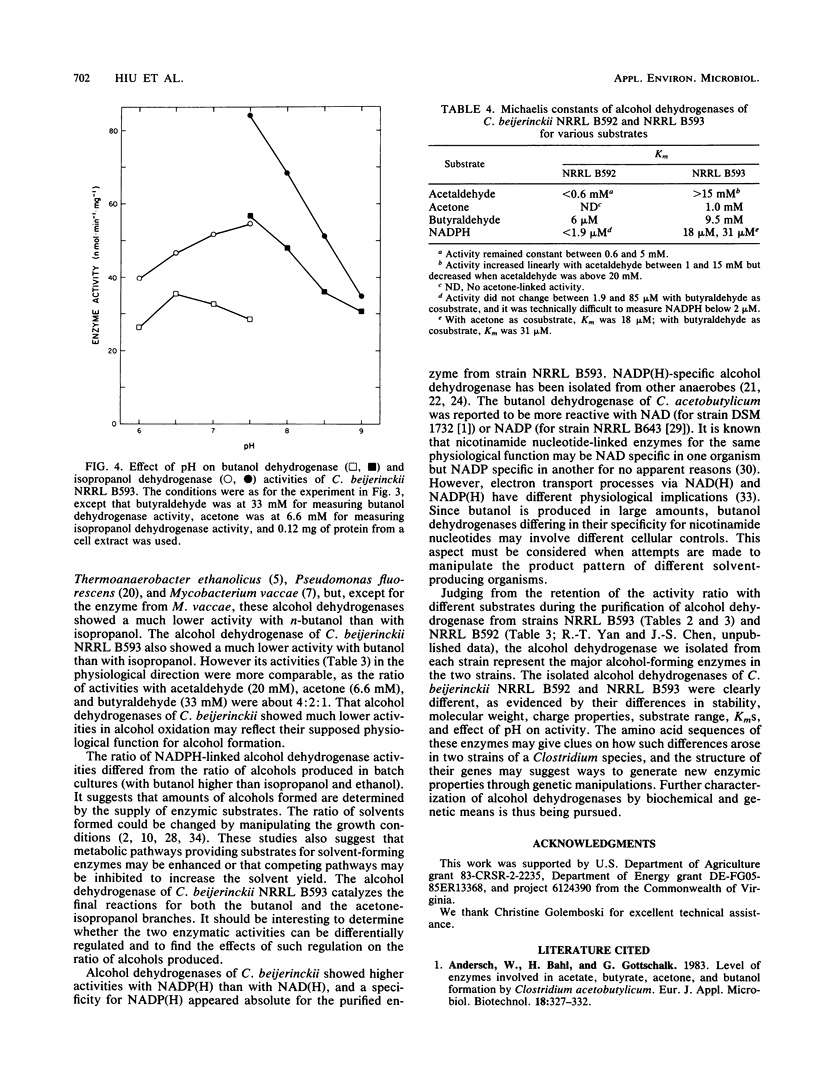
Alcohol-producing strains of Clostridium beijerinckii (Clostridium butylicum) produce, besides acetone, either n-butanol and ethanol or n-butanol, ethanol, and isopropanol as their characteristic products. Alcohol dehydrogenase has been isolated from a strain (NRRL B593) of C. beijerinckii producing isopropanol and from a strain (NRRL B592) not producing isopropanol. Butanol-ethanol dehydrogenase activities were present in both strains, but isopropanol dehydrogenase activity was present only in the isopropanol-producing strain. The butanol-ethanol dehydrogenase of strain NRRL B592 had Mr 66,000 and a Km of 6 μM for butyraldehyde. In contrast, the butanol-ethanol-isopropanol dehydrogenase of strain NRRL B593 had a Mr 100,000 and Kms of 9.5 and 1.0 mM for butyraldehyde and acetone, respectively. In a purification by four different types of separatory methods (DEAE-cellulose, hydroxyapatite, Sephacryl S-300, and Matrex Gel Red A), butanol-ethanol-isopropanol dehydrogenase activities of strain NRRL B593 were purified up to 200-fold (10 to 30% yield), and these activities were not separated. Gel electrophoresis followed by activity stain also revealed distinct mobilities for the butanol-ethanol dehydrogenase of strain NRRL B592 and the butanol-ethanol-isopropanol dehydrogenase of strain NRRL B593. In cell extracts from both strains, a higher alcohol dehydrogenase activity was measured with NADP(H) than with NAD(H). The 150- to 200-fold-purified alcohol dehydrogenase from strain NRRL B593 did not show any NAD(H)-linked activities. The Km for NADPH was 31 μM (with butyraldehyde as cosubstrate) and 18 μM (with acetone as cosubstrate) for the alcohol dehydrogenase of strain NRRL B593. This study showed that the alcohol dehydrogenases from two strains of C. beijerinckii differed significantly.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl H., Gottwald M., Kuhn A., Rale V., Andersch W., Gottschalk G. Nutritional Factors Affecting the Ratio of Solvents Produced by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):169–172. doi: 10.1128/aem.52.1.169-172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryant F., Ljungdahl L. G. Characterization of an alcohol, dehydrogenase from Thermoanaerobacter ethanolicus active with ethanol and secondary alcohols. Biochem Biophys Res Commun. 1981 May 29;100(2):793–799. doi: 10.1016/s0006-291x(81)80244-6. [DOI] [PubMed] [Google Scholar]
- Coleman J. P., Perry J. J. Purification and characterization of the secondary alcohol dehydrogenase from propane-utilizing Mycobacterium vaccae strain JOB-5. J Gen Microbiol. 1985 Nov;131(11):2901–2907. doi: 10.1099/00221287-131-11-2901. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Datta R., Zeikus J. G. Modulation of acetone-butanol-ethanol fermentation by carbon monoxide and organic acids. Appl Environ Microbiol. 1985 Mar;49(3):522–529. doi: 10.1128/aem.49.3.522-529.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Chen J. S. Acidic Conditions Are Not Obligatory for Onset of Butanol Formation by Clostridium beijerinckii (Synonym, C. butylicum). Appl Environ Microbiol. 1983 Aug;46(2):321–327. doi: 10.1128/aem.46.2.321-327.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Johnson J. L., Moore W. E., Holdeman L. V., Chen J. S. Acetone, Isopropanol, and Butanol Production by Clostridium beijerinckii (syn. Clostridium butylicum) and Clostridium aurantibutyricum. Appl Environ Microbiol. 1983 Mar;45(3):1160–1163. doi: 10.1128/aem.45.3.1160-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald M., Hippe H., Gottschalk G. Formation of n-Butanol from d-Glucose by Strains of the "Clostridium tetanomorphum" Group. Appl Environ Microbiol. 1984 Sep;48(3):573–576. doi: 10.1128/aem.48.3.573-576.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laskin A. I., Barist I., Barnabe N. Thermostable NAD-linked secondary alcohol dehydrogenase from propane-grown Pseudomonas fluorescens NRRL B-1244. Appl Environ Microbiol. 1983 Jul;46(1):98–105. doi: 10.1128/aem.46.1.98-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Barnabe N., Marczak I. Stereospecificity and other properties of a novel secondary-alcohol-specific alcohol dehydrogenase. Eur J Biochem. 1981 Oct;119(2):359–364. doi: 10.1111/j.1432-1033.1981.tb05616.x. [DOI] [PubMed] [Google Scholar]
- Hsu E. J., Ordal Z. J. Comparative metabolism of vegetative and sporulating cultures of Clostridium thermosaccharolyticum. J Bacteriol. 1970 May;102(2):369–376. doi: 10.1128/jb.102.2.369-376.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D. E., Johnston M. Purification and properties of a secondary alcohol dehydrogenase from the parasitic protozoan Tritrichomonas foetus. J Biol Chem. 1985 Jul 5;260(13):8038–8043. [PubMed] [Google Scholar]
- Kokesh F. C., Westheimer F. H. A reporter group at the active site of acetoacetate decarboxylase. II. Ionization constant of the amino group. J Am Chem Soc. 1971 Dec 29;93(26):7270–7274. doi: 10.1021/ja00755a025. [DOI] [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monot F., Martin J. R., Petitdemange H., Gay R. Acetone and Butanol Production by Clostridium acetobutylicum in a Synthetic Medium. Appl Environ Microbiol. 1982 Dec;44(6):1318–1324. doi: 10.1128/aem.44.6.1318-1324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson B. O., Fathi-Afshar S., Rudd D. F., Lightfoot E. N. Biomass as a source of chemical feedstocks: an economic evaluation. Science. 1981 Jul 31;213(4507):513–517. doi: 10.1126/science.213.4507.513. [DOI] [PubMed] [Google Scholar]


