Abstract
During brain development, an intricate array of signals is likely to control the transition from proliferation to differentiation, particularly in the complex cerebral cortex. Although factors regulating proliferation and differentiation have been identified, little is known about mechanisms governing the exit of precursors from the cell cycle. We now report that pituitary adenylate cyclase-activating polypeptide (PACAP), a new member of the vasoactive intestinal peptide family expressed in embryonic brain, promotes this transition. In virtually pure cultures of embryonic day 13.5 (E13.5) rat cortical precursors, PACAP inhibited [3H]thymidine incorporation by 43%, decreasing the proportion of mitotic cells. Moreover, the peptide promoted morphological and biochemical differentiation; PACAP elicited a 2-fold increase in cells bearing neurites and a 30% increase in neurotrophin trkB receptor expression, indicating that PACAP induced cell cycle withdrawal and promoted neuronal differentiation. The expression of PACAP ligand and receptor in precursors raised the possibility of autocrine function. Indeed, 85% of cells exhibited PACAP immunoreactivity while 64% expressed type I receptor, which, in turn, mediated cAMP activation and phosphorylated cAMP response element binding protein nuclear signaling. Furthermore, treatment with the PACAP antagonist or neutralizing antibody increased DNA synthesis and proliferation, which is consistent with interruption of ongoing mitotic inhibition mediated by endogenous PACAP. Our observations suggest that cortical precursors produce PACAP as an autocrine signal to elicit cell cycle withdrawal, inducing the transition from proliferation to neuronal differentiation.
Keywords: neurogenesis, proliferation, neuronal differentiation, neuropeptide, basic fibroblast growth factor
The cerebral cortex is comprised of layers of neurons exhibiting distinct morphologies and synaptic connections (1, 2). Developmental studies suggest that the exit of precursors from the mitotic cycle is a critical event for establishing neuron phenotype and patterning because laminar fate apparently is determined during this process (3). Although precursor withdrawal from the cell cycle may result from the diminishing action of proliferative factors, the sustained expression of endogenous mitogens in the developing cortex (4–6) suggests that inhibitory signals are required to elicit cell cycle exit. Although molecules stimulating proliferation and differentiation have been identified (4, 7–9), little is known about mitotic inhibitors.
The 38-amino acid peptide pituitary adenylate cyclase-activating polypeptide (PACAP) (10), a new member of the vasoactive intestinal peptide (VIP)/secretin/glucagon family (11–13), is expressed in brain as early as embryonic day 14 (E14). Although peptide levels in whole brain increase 5-fold by birth with little change thereafter, mature expression is mainly restricted to the diencephalon and limbic structures, reflecting PACAP’s role in hypothalamic–pituitary regulation (10, 11). The early and progressive prenatal expression of PACAP raises the possibility that it plays a role in cortical neurogenesis. Peptide receptors also are present in developing brain and are classified as two types (13, 14). Type I receptors are PACAP-preferring, binding PACAP at nanomolar concentrations while binding VIP in the micromolar range (14). In contrast, type II receptors exhibit nanomolar affinities for both peptides (15, 16). In the present study, we have defined the effects of PACAP on embryonic rat cortical precursors in culture. PACAP inhibits precursor mitosis and enhances neuronal differentiation, thus serving as a signal triggering the transition from proliferation to differentiation. The ligand–receptor system’s expression and function suggest that PACAP plays an autocrine role. Consequently, cortical precursors may produce PACAP to elicit cell cycle withdrawal, a process in which laminar fate is determined (3).
METHODS
Cultures.
Time-mated, pregnant Sprague Dawley rats were obtained from Hilltop Labs (Philadelphia). After killing E13.5, embryo skull and meninges were removed, and cerebral cortices were isolated, mechanically dissociated, and plated (105 cells) on poly-d-lysine (0.1 mg/ml)-coated 35-mm dishes or 24-multiwell plates in defined medium (no insulin) as described (17, 18). In proliferation studies only, medium contained 10% heat-inactivated fetal bovine serum. Cultures were maintained in a humidified 5% CO2/air incubator. Vehicles, PACAP, growth factors (in 10 μg/ml BSA), or a cAMP pathway activator or analog [forskolin in 0.3% ethanol, dibutyryl-cAMP (Db-cAMP) in H2O] was added to cultures at plating.
DNA Synthesis.
Incorporation of [3H]thymidine ([3H]dT) was used to assess DNA synthesis, a marker for cells in S-phase of the mitotic cycle (17, 18). Cells were incubated with [3H]dT [2 μCi/ml (1 Ci = 37 GBq), 79 Ci/mmol; NEN] during the final 2 h of 6-h or final 4 h of 24-h cultures, respectively. Incorporation was assayed by scintillation spectroscopy (18). Unless stated otherwise, experiments were performed three or more times, with three to four samples/group/experiment.
Immunocytochemistry.
After 4% paraformaldehyde fixation (20 min), immunocytochemistry was performed with antibodies to MAP2 (1:1,000; I. Fischer, Medical College of Philadelphia), NSE (1:1,000; Polysciences), glial fibrillary acidic protein (1:1,000; Dako), RC1 (R. Schwarting, Dana–Farber Cancer Institute), myelin basic protein (1:500; D. Colman, Mt. Sinai Medical School), trkB (1:1000; Santa Cruz Biotechnology and D. Kaplan, Montreal University), PACAP, and type I receptor (1:5000 and 1:1000; A. Arimura, Tulane University) as described (17, 18). Staining was visualized using a Vectastain ABC Kit (Vector Laboratories). All studies included a no primary antibody control and assessment of positive- and negative-staining cells to evaluate specificity. For phosphorylated cAMP response element binding (P-CREB) protein detection, 24-h cultures were stimulated with PACAP (10 nM) or vehicle for 15 min, fixed, and processed as described (anti-P-CREB, 1:1000; D. Ginty, Harvard University) (19). PACAP antibody specificity was assessed by preincubating antiserum with 17 μM PACAP, VIP, secretin, or peptide–His–Ileu 18 h before use. Glial marker antibodies were active because signal was detected in astrocyte and oligodendrocyte cultures.
To visualize cells synthesizing DNA, cultures were exposed to BrdUrd (10 μM) during the final 2 or 4 h of incubation (20). After fixation, cells were exposed to 2 N HCl (30 min), rinsed twice in PBS, incubated for 1 h (21°C) in monoclonal anti-BrdUrd (1:50, Dako) in PBS/0.3% Triton X-100, and visualized as above. The labeling index, defined as the proportion of total cells incorporating BrdUrd into the nucleus, was determined by scoring 200 cells from randomly selected, nonoverlapping fields in each of three dishes per group per experiment (18).
To define relationships of mitosis to differentiation, cells were exposed to BrdUrd for the first 3 h of incubation and then washed three times with BrdUrd-free medium. Cultures were fixed either immediately or after a further 21-h incubation in BrdUrd-free medium. Cells were stained for MAP2 using the ABC peroxidase method as above and subsequently for BrdUrd incorporation, which was visualized using a fluorescein isothiocyanate-conjugated secondary antibody (1:100; Vector Laboratories). Photographic images, obtained using a Leitz Aristoplan microscope, were processed using Adobe photoshop on a Macintosh computer.
Cell and Neurite Counting.
Living cells in three randomly selected 1-cm strips [(3% of area)/dish, 3–4 dishes/group] were counted over 3 days under phase microscopy (17, 18). Zero-time cell counts were obtained 3 h after plating. For the 6-h incubation paradigm, cells were counted at 3 and 6 h in three independent experiments containing three dishes/group. Cells bearing neurites ≥2 cell soma diameters were assessed in living cultures between 24 and 48 h, evaluating 3% of the area.
Radioimmunoassay cAMP.
Twenty four-hour cultures were treated with PACAP (10−12−10−6 M) in the presence of 0.5 mM isobutylmethylxanthine for 15 min and extracted and lyophilized (21). cAMP content was assayed using an Amersham RIA kit.
PACAP.
Freshly dissected tissue was collected, frozen on dry ice, and stored at −80°C until assay was performed by Dr. A. Arimura (12).
Statistics.
Raw data from multiple experiments were grouped for statistical analysis by Student’s t test, ANOVA (statview 4.1), or ANOVA with repeated measures (superanova) on a Macintosh computer. For presentation after statistical analysis, data from each experiment were normalized to the mean of its control, and grouped data are expressed as percentage of control.
Materials.
PACAP, PACAP6–38, peptide–His–Ileu, VIP, and secretin were from the American Peptide Company (Santa Clara, CA); BDNF, NT3, and NGF were gifts of Cephalon (West Chester, PA); Db-cAMP, forskolin, isobutylmethylxanthine, and BrdUrd were from Sigma; and media were from GIBCO.
RESULTS
Characterization of Cortical Precursors in Culture.
To define the effects of regulatory factors, cortical precursors from E13.5 rat embryos were cultured in defined medium. At 3 h, half of the cells expressed neuronal markers, MAP2 [56 ± 3% (mean ± SEM)], and NSE (55 ± 2.3%), and the proportions increased at 24 h (MAP2 = 93 ± 1.2%; NSE = 90 ± 1.5%) and were 100% by 48 h (Fig. 1). In contrast, less than 1% of the cells expressed the astrocyte marker glial fibrillary acidic protein, and no cells expressed the oligodendrocyte antigens RC1 and myelin basic protein, indicating that the cultures contained a virtually pure population of differentiating neuronal precursors.
Figure 1.
Characterization of E13.5 cortical precursor cultures. Cells were incubated for 3 (a, b, d, e) or 24 h (c and f) and examined by phase (a and d) or brightfield microscopy to detect neuronal markers, immunoreactive MAP2 (b and c), and NSE (e and f). Neuronal markers increase from ≈50% at 3 h (b and e) to ≈90% at 24 h (c and f) (positive staining indicated by arrows; arrowhead = negative cells). Cells were unstained when primary antibody was omitted. MAP2 and NSE analyses were performed on five and two experiments, respectively. (Bar = 50 μm.)
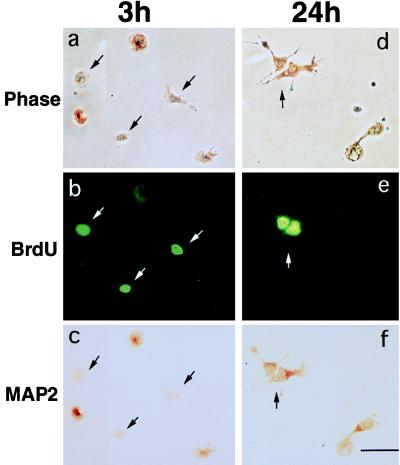
In vivo, young neurons undergo differentiation only after precursors have ceased proliferating in the ventricular zone (1, 2). To define relationships in culture, we identified cells in the mitotic cycle and characterized expression of MAP2 using double immunostaining. Nuclear incorporation of BrdUrd into newly synthesized DNA serves as an index of cells in S-phase of the mitotic cycle (20). Immediately after a 3-h exposure to BrdUrd, only 10 ± 2.5% (mean ± SEM) of BrdUrd-labeled cells were MAP2-positive, suggesting that precursors engaged in mitotic S-phase do not express differentiated traits (Fig. 2, a–c). In contrast, when cultures initially exposed to BrdUrd were washed and incubated in BrdUrd-free medium until 24 h, 67 ± 4% of BrdUrd-labeled cells were MAP2-positive (Fig. 2, d–f), indicating that, after mitosis, precursors differentiated, recapitulating the in vivo ontogenetic sequence.
Figure 2.
Cortical precursors differentiate after proliferation. Cells were exposed to BrdUrd for 3 h after plating and were fixed either immediately (a–c) or after further incubation in BrdUrd-free medium until 24 h (d–f). The cultures were processed for double immunostaining for BrdUrd and MAP2. Cells were examined under phase (a and d), epifluorescence (b and e), and brightfield (c and f) microscopy. (a–c) BrdUrd-labeled precursors (arrows) fixed at 3 h did not express MAP2, suggesting that mitosis and differentiation do not occur at the same time. (d–f) BrdUrd-positive cells subsequently expressed MAP2 at 24 h (arrow), indicating that, after mitosis, cortical precursors differentiated. Data were from two experiments, n = 9. (Bar = 50 μm.)
Finally, with advancing time, fewer cells were mitotic in vitro, as the BrdUrd labeling index decreased from 25 ± 1.5% (mean ± SEM) at 3 h to 23 ± 0.3% at 6 h to 13 ± 0.3% at 24 h. Thus, cultured precursors underwent sequential division and differentiation and exhibited a progressive reduction in mitosis, providing a model system to define underlying regulation.
PACAP Induces Precursors to Withdraw from the Cell Cycle.
Previous studies demonstrate that PACAP and its type I receptor are expressed in the developing brain (10). To define potential roles in cortex, we first examined PACAP expression and receptor activation. In freshly dissected cortex tissue, significant quantities of PACAP were detected (Table 1). To determine whether precursors possess functional receptors, peptide activation of intracellular signaling was examined. We measured cAMP levels after peptide treatment because currently defined receptors couple to this pathway (11, 13–16). PACAP elicited a 5-fold increase in cAMP levels, with a plateau at 0.1 nM, indicating that precursors possess a PACAP response system (Fig. 3). Peptides are frequently unstable in defined culture media, so a supramaximal PACAP concentration (10 nM) was used in subsequent studies.
Table 1.
PACAP expression in neural tissues
| Tissue | Protein, pg/mg* |
|---|---|
| Cerebral cortex (E14.5) | 480 ± 15 |
| Superior cervical ganglion (E15.5) | 1101 ± 117 |
| Liver (adult) | none detected |
*Mean ± SEM.
Figure 3.
PACAP elicits intracellular signaling in precursors. Twenty- four-hour cultures were treated with increasing concentrations of PACAP for 15 min. PACAP increased cAMP levels in a dose-dependent manner, with a plateau at 0.1 nM, indicating that precursors express functional receptors.
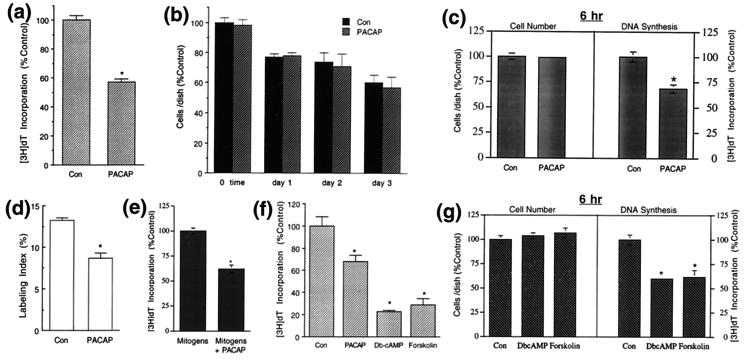
In precursor cultures, PACAP (10 nM) elicited a 43% decrease in [3H]dT incorporation at 24 h (Fig. 4a). Smaller inhibitory effects were obtained at 1 nM (24 ± 5%; n = 17; P < 0.002), with a trend toward inhibition observed at 0.1 nM. Although the reduction in [3H]dT incorporation may have represented fewer cells entering S-phase of the cell cycle, other mechanisms may have been involved, including toxicity or differential cell plating/survival. However, PACAP exposure did not decrease cell numbers over 3 days compared with the control (Fig. 4b), suggesting that PACAP was not a general cellular toxin. Nonetheless, over several days with ongoing cell loss, potential toxicity may be masked by differential survival; specifically, PACAP may have elicited selective death of mitotic precursors balanced by enhanced plating efficiency or survival of nonmitotic cells. To clarify this issue, we altered the paradigm to minimize changes in population size; precursors were cultured for 6 h only. To determine whether PACAP elicited differential plating or survival, we first performed counts at 3 h, when cells had attached. There was no difference between control and PACAP-treated groups [control = 69933 ± 1858, PACAP = 68100 ± 1514 (cells/dish ± SEM), P < 0.5], indicating that PACAP did not alter cell plating or survival. At 6 h, the precursors appeared healthy, and there was no difference in cell number compared with that plated initially (control 3 h = 69933 ± 1858, 6 h = 66900 ± 2000, P < 0.3; PACAP 3 h = 68100 ± 1514, 6 h = 64400 ± 1404, P < 0.2), indicating minimal ongoing cell death at this time. Because cells were not dying, there was no subpopulation for PACAP to potentially rescue. Moreover, peptide exposure did not decrease cell number at 6 h, so PACAP was not toxic (Fig. 4c). Nevertheless, in the absence of differential plating/survival or toxicity, PACAP inhibited [3H]dT incorporation by 30% (Fig. 4c) and reduced the labeling index by 25% (labeling index: control = 23 ± 0.3%, PACAP = 17 ± 0.4%; n = 7; P < 0.0001) at 6 h, comparable to inhibitory effects at 24 h (Fig. 4, a and d), suggesting that PACAP induced precursors to leave the cell cycle.
Figure 4.
PACAP inhibits precursor mitosis. (a) At 24 h, PACAP (10 nM) inhibited [3H]dT incorporation by 43%. Data are expressed as percentage control ± SEM. Data were from seven experiments (hereafter indicated as “# expts”), four wells per group; n = 28. Control cpm = 534-1508. ∗, Differs from control at P < 0.02. (b) PACAP did not elicit decreased cell numbers compared with respective controls over 3 days (3 expts); n = 9. (c) At 6 h, although PACAP did not elicit decreased cell number, the peptide inhibited [3H]dT incorporation, indicating that the inhibitory effect was not due to toxicity. Data are expressed as percentage control ± SEM; n = 8; ∗, P < 0.02. (d) PACAP decreased the proportion of cells engaged in the mitotic cycle at 24 h (labeling index = BrdUrd-positive cells per total). Data are expressed as mean percentage ± SEM. ∗, P < 0.001. (e) PACAP inhibited [3H]dT incorporation in the presence of endogenous mitogens at 24 h [insulin (10 μg/ml) or IGF1 (10 nM), bFGF (20 ng/ml), and epidermal growth factor (20 ng/ml)] (3 expts); n = 10. Mitogens cpm = 3204–4700. ∗, P < 0.01. (f) Db-cAMP (1 mM) and forskolin (30 μM) inhibited [3H]dT incorporation at 24 h, producing similar though greater effects than PACAP (2 expts); n = 8; ∗, P < 0.001. (g) At 6 h, although DbcAMP and forskolin did not elicit decreased cell numbers, the drugs inhibited [3H]dT incorporation, indicating that the inhibitory effects were not due to toxicity; n = 8; ∗, P < 0.0004. Con, control.
Because precursor mitosis in vivo may be stimulated by endogenous mitogens, such as insulin-like growth factors (IGF), basic fibroblast growth factor (bFGF), and epidermal growth factor (4, 7–9, 22), we examined PACAP effects in their presence. PACAP blocked stimulation elicited by the combined factors (Fig. 4e) and that elicited by IGF alone [IGF = 100 ± 4%, IGF + PACAP = 64 ± 5%, (percentage control ± SEM), P < 0.03] or bFGF alone (bFGF = 100 ± 3%, bFGF + PACAP = 72 ± 2%, P < 0.007), suggesting that PACAP provides a general signal to induce cell cycle withdrawal. In contrast, the neurotrophins (NT3, BDNF, NGF) survival and differentiation factors expressed in developing cortex (7, 8, 23–27) did not affect DNA synthesis (data not shown), suggesting that PACAP plays a distinct role (note: trophic activity was exhibited by NT3 and NGF in sympathetic, and BDNF in cerebellar granule, cultures).
PACAP effects were highly specific; related peptides VIP, secretin, and peptide–His–Ileu at 1 nM did not inhibit mitosis (data not shown). However, 1 μM VIP reproduced mitogenic inhibition elicited by 1 nM PACAP [control = 100 ± 1%, VIP = 68 ± 2% (percentage control ± SEM), P < 0.0001, n = 10], consistent with peptide actions via the PACAP type I receptor (13, 14).
To evaluate the role of cAMP, we used a cAMP analog or activator, Db-cAMP, and forskolin. At 24 h, the drugs inhibited [3H]dT incorporation up to 75% (Fig. 4f). To evaluate possible changes in cell plating or death, we used the 6-h paradigm: although the drugs did not alter cell number, they inhibited [3H]dT incorporation >40% (Fig. 4g), suggesting that PACAP elicits cell cycle exit through the cAMP pathway.
PACAP Enhances Cortical Precursor Differentiation.
As a factor arresting growth, PACAP may serve to initiate the transition from proliferation to differentiation. Consequently, we characterized PACAP effects on biochemical and morphological markers of differentiation. BDNF and its trkB receptor are widely expressed in developing cortex (25–27), promoting differentiation and survival (8, 23). Initially undetectable at E14, trkB expression in vivo increases progressively with development (24). In precursor cultures, only a subpopulation expressed trkB, detected using two independent antibodies. PACAP treatment increased trkB immunoreactive cells by 30% (Fig. 5, a–c), suggesting that PACAP enhanced receptor expression. Furthermore, in cultures in which PACAP decreased mitosis, the peptide increased the number of cells with neurites 2-fold, indicating that mitotic inhibition was accompanied by enhanced differentiation (Fig. 5, d–f). Finally, cAMP pathway activation similarly, though more robustly, enhanced the number of cells with neurites (Fig. 5g) without altering cell number (data not shown). In sum, these observations suggest that PACAP, acting via the cAMP cascade, triggers the transition from precursor proliferation to neuronal differentiation.
Figure 5.
PACAP enhances cortical precursor differentiation. Precursors were incubated in control or PACAP-containing medium and were analyzed for trkB expression (a–c) and neurite outgrowth (d–f). (a) Brightfield photomicrograph of trkB immunoreactive precursors at 24 h: only a subset express trkB, localized to cytoplasm (arrows; arrowhead = negative cell; Santa Cruz Biotechnology antiserum). (b) Similar staining was observed with a different antiserum (D. Kaplan, Montreal University). (c) PACAP increased trkB-positive cells by 30%. Data are expressed as percentage control ± SEM. Range of positive cells: control = 36–67%; PACAP = 57–84% (4 expts using both antisera); n = 14; ∗, P < 0.01. (d and e) Phase photomicrographs of control (d) and PACAP-treated cultures at 48 h (e). (f) PACAP doubled the percentage of cells with neurites that were ≥2 cell soma diameters. Data are expressed as mean ± SEM (5 expts); n = 15; ∗, P < 0.007. (g) DbcAMP and forskolin elicited a 4-fold increase in neurite-bearing cells (2 expts); n = 6; ∗, P < 0.001. (Bar = 50 μm.) Con, control.
PACAP Is an Autocrine Inhibitor of Precursor Mitosis.
Because PACAP expression in the embryonic cortex (Table 1) precedes synapse formation, we hypothesized that precursors produce PACAP themselves to elicit cell cycle withdrawal, in an autocrine/paracrine fashion. At 24 h, 85 ± 1.6% of cells exhibited PACAP immunoreactivity, suggesting that most precursors can synthesize PACAP (Fig. 6a). Furthermore, two lines of evidence suggest that the majority of cells possess type I receptors. First, 64 ± 1.7% of cells stained with a PACAP type I receptor antiserum raised against the receptor 25 residue C-terminal fragment (M. Li, T. Kozicz, Y. Shuto, A. Somogyvari-Vigh, S. Vigh, H. Onda, D. Hurley, and A. Arimura, unpublished data) (Fig. 6b). Second, a similar proportion of cells exhibited intracellular signaling in response to PACAP. Because PACAP stimulates cAMP levels in precursors (Fig. 3), we examined a transcription factor downstream of cAMP, P-CREB (19). After PACAP exposure, 62 ± 3% of cells expressed nuclear P-CREB immunoreactivity (Fig. 6, c and d), corroborating receptor staining data and suggesting that PACAP elicits nuclear signaling through receptor binding.
Figure 6.
PACAP is an autocrine factor. (a) At 24 h, 85% of precursors expressed intense PACAP immunoreactivty localized to the cytoplasm (arrow). Staining was blocked by antibody preincubation with PACAP but not related peptides, secretin and peptide–His–Ileu (not shown). (Arrowhead = negative cell) (5 expts). (b) At 24 h, 64% of cells exhibited immunoreactivity with a PACAP type I receptor antiserum (2 expts). (c and d) Detection of PACAP-responsive precursors by examining P-CREB immunoreactivity in 24 h cultures. Fifteen minutes after PACAP exposure, nuclear P-CREB staining was detected in 62 ± 3% of precursors (d) whereas only 2.3 ± 0.3% of cells exhibited signals in the vehicle-treated control (c) (5 expts). (e) Blockade of endogenous PACAP function increases [3H]dT incorporation. Cells were incubated for 24 h in control medium or in medium containing peptide antagonist PACAP6–38 (100 nM). Antagonist-induced stimulation was reversed by coincubation with PACAP (3 expts); n = 11; ∗, P < 0.004. (f) PACAP-neutralizing antiserum increased [3H]dT incorporation. Precursors were incubated in control medium containing nonimmune rabbit serum or medium containing PACAP antiserum (1:3000) for 24 h (2 expts); n = 8; ∗, P < 0.016. (g) In serum containing medium, cell growth was observed at 24 h only in the presence of PACAP antagonist (100 nM) but not PACAP peptide (10 nM), suggesting that endogenous PACAP inhibits proliferation. Data are expressed as mean cells per dish ± SEM (2 expts); n = 6; ∗, P < 0.009. (Bar = 50 μm.) Con, control.
The high proportions of cells exhibiting peptide and type I receptor immunoreactivity indicated that at least 50% expressed both components, raising the possibility of a functional autocrine circuit, in which released PACAP tonically inhibited ongoing mitosis. The specific PACAP antagonist, amino acids 6–38 of the peptide (PACAP6–38), has been well characterized (28). In cortical cultures, the antagonist stimulated [3H]dT incorporation 30% (Fig. 6e), consistent with interruption of ongoing inhibition mediated by endogenous PACAP. Moreover, antagonist stimulation was reversed by coincubation with PACAP, confirming specificity. Similar blockade of endogenous PACAP activity was observed with peptide-neutralizing antibody (12) (Fig. 6f). Finally, in addition to DNA synthesis, we examined effects on proliferation, using enriched culture conditions: The PACAP antagonist elicited a 16% increase in the number of cells over that plated initially whereas proliferation was completely prevented by peptide exposure (Fig. 6g). In sum, these data support the contention that PACAP serves as an autocrine inhibitor of precursor proliferation, inducing progressive cell cycle withdrawal. However, although endogenous PACAP may tonically inhibit ongoing mitosis, its role in differentiation remains unclear because the antagonist did not block baseline neurite outgrowth.
DISCUSSION
Our observations suggest that PACAP, produced by cortical precursors, serves as an autocrine mitotic inhibitor, eliciting the transition from proliferation to differentiation. To define PACAP activity, we used a dissociated cell culture model in which precursors undergo sequential mitosis and differentiation. Although precursors remained proliferative for at least 24 h, progressively fewer cells were mitotic with time, suggesting action of endogenous mitotic inhibitory signals. The low density conditions allowed definitive quantification of cell loss, production, and morphological and immunocytochemical characteristics, a difficult task in reaggregate cultures. Furthermore, low density cultures may have augmented effects of exogenous factors that normally function in autocrine fashion because PACAP was less effective after cell reaggregation (not shown). However, PACAP activities defined in culture need to be tested in vivo.
Our observations indicate that the embryonic cortex contains both PACAP and its receptor. In culture, PACAP treatment inhibited cortical precursor DNA synthesis. PACAP inhibited mitosis without inducing cell death or promoting differential plating or survival, suggesting that the peptide induced precursors to exit the cell cycle. Indeed, fewer cells were engaged in mitotic S-phase after PACAP exposure. Furthermore, PACAP inhibited stimulation elicited by endogenous cortical mitogens, suggesting that PACAP serves as a general signal to cease proliferation. However, the possibility that the mitogenic factors and PACAP act on different subpopulations will require further study by receptor colocalization methods.
It is possible that PACAP acts indirectly to alter DNA synthesis, affecting survival or function of nonmitotic cells that in turn act on mitotic precursors. However, this seems unlikely because double-labeling studies indicate that 95% of BrdUrd-labeled mitotic precursors exhibit PACAP receptors (N.L. and E.D.-B., unpublished results) compatible with direct action.
Although PACAP inhibition of mitosis did not appear to be the result of toxicity, cell numbers were not reduced at later times, as might be expected. Did PACAP induce mitotic cell death on the one hand, and promote survival of differentiating cells on the other? Although formally possible, we do not favor this mechanism for several reasons: (i) It is highly uncharacteristic that a survival factor would fail to increase cell number over control at any time, from 3 h to 3 days; (ii) it seems highly unlikely that three agents, PACAP, forskolin, and Db-cAMP, evaluated at two time points (6 and 24 h) would elicit different degrees of inhibition (25–75%) or cell loss that were coordinately balanced by enhanced survival, yielding no change in cell number; and (iii) for forskolin specifically, the magnitude of enhanced survival that would account for a 28% increase in differentiated cells far exceeded the 70% reduction in the pool of mitotic cells (labeling index = 13% at 24 h), and would have yielded a cell number increase that was not observed.
Alternatively, cell numbers did not change as a result of unmet trophic requirements and ongoing autocrine mitotic inhibition. Precursor mitosis, which decreased from 23 (labeling index, 6 h) to 13% (24 h), was further inhibited 30–43% by PACAP, representing ≈6% reduction in the total cell population, a small change compared with the ≈22% cell death over 24 h. Confounding interactions between precursor mitosis and survival have been observed previously in models using defined media, suggesting requirements for as yet undefined trophic factors (4, 17, 18). Indeed, the addition of serum, a source of trophic factors, to the cultures entirely prevented cell loss over 24 h and permitted proliferation when the PACAP antagonist was present. Finally, the ongoing inhibitory effects of endogenous, autocrine PACAP in control groups likely minimized differences elicited by exogenous PACAP. Although extracellular PACAP antagonists did augment mitosis, possible intracellular actions may require blockade by antisense oligonucleotide strategies.
PACAP inhibitory effects differed markedly with the actions of another family of cortical signals, the neurotrophins. It was reported that NT3 had no effect in E14 cultures at 2 days but may decrease DNA synthesis by 4 days (7, 8). In our study, NT3, NGF, and BDNF did not altered precursor mitosis, confirming previous reports (7, 8). In contrast, PACAP increased cAMP levels and P-CREB signaling within minutes and inhibited mitosis by 6 h, suggesting that PACAP plays a role before NT3 during cortical ontogeny.
Although PACAP and VIP are related molecules, they serve distinct functions. Studies of mouse embryos in culture and in utero suggest that VIP stimulates proliferation and survival during neurogenesis, an activity not shared by PACAP (29, 30). Furthermore, although VIP is apparently derived from the mother (31) and acts through cAMP-independent pathways (29), PACAP is expressed by cortical precursors in vitro and in vivo (10) and acts via PACAP type I receptors and the cAMP cascade, supporting peptide-specific functions.
Although intracellular messengers remain undefined, our studies indicating that PACAP increases cAMP levels and that cAMP pathway activation reproduces peptide effects provide clues to inhibitory mechanisms. Potentially, cAMP pathways directly regulate cell cycle components, repressing G1-specific cyclin genes (32) or augmenting cyclin-dependent kinase inhibitors p16, p21, and p27 (33, 34). Alternatively, the cAMP cascade may serve as a molecular gate, regulating signal flow through the tyrosine kinase receptors of cortical mitogens (35).
PACAP inhibition of precursor mitosis and stimulation of neurite outgrowth and trkB expression suggest that the peptide serves as a signal triggering the transition from proliferation to differentiation. Potentially, precursor proliferation depends on integrating competing mitogenic regulators, balancing stimulatory factors expressed in cortex, including bFGF, epidermal growth factor, and IGFs and inhibitors such as PACAP or, at later stages, γ-aminobutyric acid and glutamate (36). The dynamic interplay of positive and negative regulators would determine the timing of cell cycle withdrawal.
The high proportions of precursors expressing PACAP ligand and active type I receptors raised the possibility of a functional autocrine/paracrine circuit, in which PACAP tonically inhibited ongoing mitosis. The increase in DNA synthesis elicited by inhibiting PACAP activity was consistent with this model, suggesting that autocrine activity was responsible for the progressive reduction in mitosis observed in vitro. More importantly, new cell production occurred only when endogenous peptide activity was blocked, suggesting that precursors produce PACAP to elicit cell cycle withdrawal. On the other hand, PACAP antagonists failed to decrease neurite outgrowth, suggesting that other autocrine signals also may regulate differentiation, such as NT3 or BDNF (7, 23). Significantly, the marked increase in neurite-bearing cells elicited by forskolin suggests that cAMP pathways play important roles in differentiation.
Our observations in culture naturally raise questions regarding the role of the PACAP ligand-receptor system in vivo. PACAP ligand and type I receptor proteins (10) and mRNAs (unpublished results) are expressed in developing brain. Furthermore, preliminary studies localize PACAP to the ventricular zone and forming cortical plate, compatible with the mitotic and differentiative effects observed in culture. Ongoing studies are focused on manipulating PACAP activity during development and defining growth factor interactions in this newly defined culture model.
Acknowledgments
We thank Drs. C. Dreyfus and P. Levitt for critical review, I. B. Black for thoughtful discussions, and M. Ramakrishnan for technical support. This work was supported by National Institute of Neurological Disorders and Stroke Grant RO1NS32401.
ABBREVIATIONS
- PACAP
pituitary adenylate cyclase-activating polypeptide
- VIP
vasoactive intestinal peptide
- E
embryonic day
- Db-cAMP
dibutyryl-cAMP
- [3H]dT
[3H]thymidine
- MAP
microtubule-associated protein
- NSE
neuron-specific enolase
- P-CREB
phosphorylated cAMP response element binding
- IGF
insulin-like growth factor
- bFGF
basic fibroblast growth factor
Note Added in Proof
Recent data indicate that VIP ligand and receptor mRNA are expressed in E14 mouse brain, suggesting that the peptide also derives from the embryo itself (37).
References
- 1.Bayer S A, Altman J. Neocortical Development. New York: Raven; 1991. [Google Scholar]
- 2.McConnell S K. Annu Rev Neurosci. 1991;14:269–300. doi: 10.1146/annurev.ne.14.030191.001413. [DOI] [PubMed] [Google Scholar]
- 3.McConnell S K, Kaznowski C E. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- 4.Drago J, Murphy M, Carroll S M, Harvey R P, Bartlett P F. Proc Natl Acad Sci USA. 1991;88:2199–2203. doi: 10.1073/pnas.88.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell P P, Finklestein S P, Dionne C A, Jaye M, Klagsbrun M. Mol Brain Res. 1991;11:71–77. doi: 10.1016/0169-328x(91)90023-q. [DOI] [PubMed] [Google Scholar]
- 6.Wanaka A, Milbrandt J, Johnson E M., Jr Development. 1991;111:455–468. doi: 10.1242/dev.111.2.455. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh A, Greenberg M E. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 8.Vicario-Abejon C, Johe K K, Hazel T G, Collazo D, McKay R D. Neuron. 1995;15:105–114. doi: 10.1016/0896-6273(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 9.Temple S, Qian X. Neuron. 1995;15:249–252. doi: 10.1016/0896-6273(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 10.Tatsuno I, Somogyvari-Vigh A, Arimura A. Peptides. 1994;15:55–60. doi: 10.1016/0196-9781(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 11.Arimura A. Regul Pept. 1992;37:287–303. [PubMed] [Google Scholar]
- 12.Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy D H, Kitada C. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 13.Christophe J. Biochim Biophys Acta. 1993;1154:183–199. doi: 10.1016/0304-4157(93)90011-c. [DOI] [PubMed] [Google Scholar]
- 14.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg P H, Journot L. Nature (London) 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 15.Lutz E M, Sheward W J, West K M, Morrow J A, Fink G, Harmar A J. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- 17.Pincus D W, DiCicco-Bloom E M, Black I B. Nature (London) 1990;343:564–567. doi: 10.1038/343564a0. [DOI] [PubMed] [Google Scholar]
- 18.DiCicco-Bloom E, Friedman W J, Black I B. Neuron. 1993;11:1101–111. doi: 10.1016/0896-6273(93)90223-e. [DOI] [PubMed] [Google Scholar]
- 19.Ginty D D, Kornhauser J M, Thompson M A, Bading H, Mayo K E, Takahashi J S, Greenberg M E. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 20.Nowakowski R S, Lewin S B, Miller M W. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 21.Pincus D W, DiCicco-Bloom E M, Black I B. Brain Res. 1990;514:355–357. doi: 10.1016/0006-8993(90)91433-h. [DOI] [PubMed] [Google Scholar]
- 22.Anchan R M, Reh T A, Angello J, Balliet A, Walker M. Neuron. 1991;6:923–936. doi: 10.1016/0896-6273(91)90233-p. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh A, Carnahan J, Greenberg M E. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 24.Maisonpierre P C, Belluscio L, Friedman B, Alderson R F, Wiegand S J, Furth M E, Lindsay R M, Yancopoulos G D. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 25.Escandon E, Soppet D, Rosenthal A, Mendoza-Ramirez J L, Szonyi E, Burton L E, Henderson C E, Parada L F, Nikolics K. J Neurosci. 1994;14:2054–2068. doi: 10.1523/JNEUROSCI.14-04-02054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein R, Parada L F, Coulier F, Barbacid M. EMBO J. 1989;8:3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knusel B, Rabin S J, Hefti F, Kaplan D R. J Neurosci. 1994;14:1542–1554. doi: 10.1523/JNEUROSCI.14-03-01542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robberecht P, Gourlet P, De Neef P, Woussen-Colle M C, Vandermeers-Piret M C, Vandermeers A, Christophe J. Mol Pharmacol. 1992;42:347–355. [PubMed] [Google Scholar]
- 29.Gressens P, Hill J M, Gozes I, Fridkin M, Brenneman D E. Nature (London) 1993;362:155–158. doi: 10.1038/362155a0. [DOI] [PubMed] [Google Scholar]
- 30.Gressens P, Hill J M, Paindaveine B, Gozes I, Fridkin M, Brenneman D E. J Clin Invest. 1994;94:2020–2027. doi: 10.1172/JCI117555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill J M, McCune S K, Alvero R J, Glazner G W, Henins K A, Stanziale S F, Keimowitz J R, Brenneman D E. J Clin Invest. 1996;97:202–208. doi: 10.1172/JCI118391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokiwa G, Tyers M, Volpe T, Futcher B. Nature (London) 1994;371:342–345. doi: 10.1038/371342a0. [DOI] [PubMed] [Google Scholar]
- 33.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 34.Kato J Y, Matsuoka M, Polyak K, Massague J, Sherr C J. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 35.Lyengar R. Science. 1996;271:461–463. doi: 10.1126/science.271.5248.461. [DOI] [PubMed] [Google Scholar]
- 36.Loturco J J, Owens D F, Heath M J, Davis M B, Kriegstein A R. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 37.Waschek J A, Ellison J, Bravo D T, Handley V. J Neurochem. 1996;66:1762–1765. doi: 10.1046/j.1471-4159.1996.66041762.x. [DOI] [PubMed] [Google Scholar]