Abstract
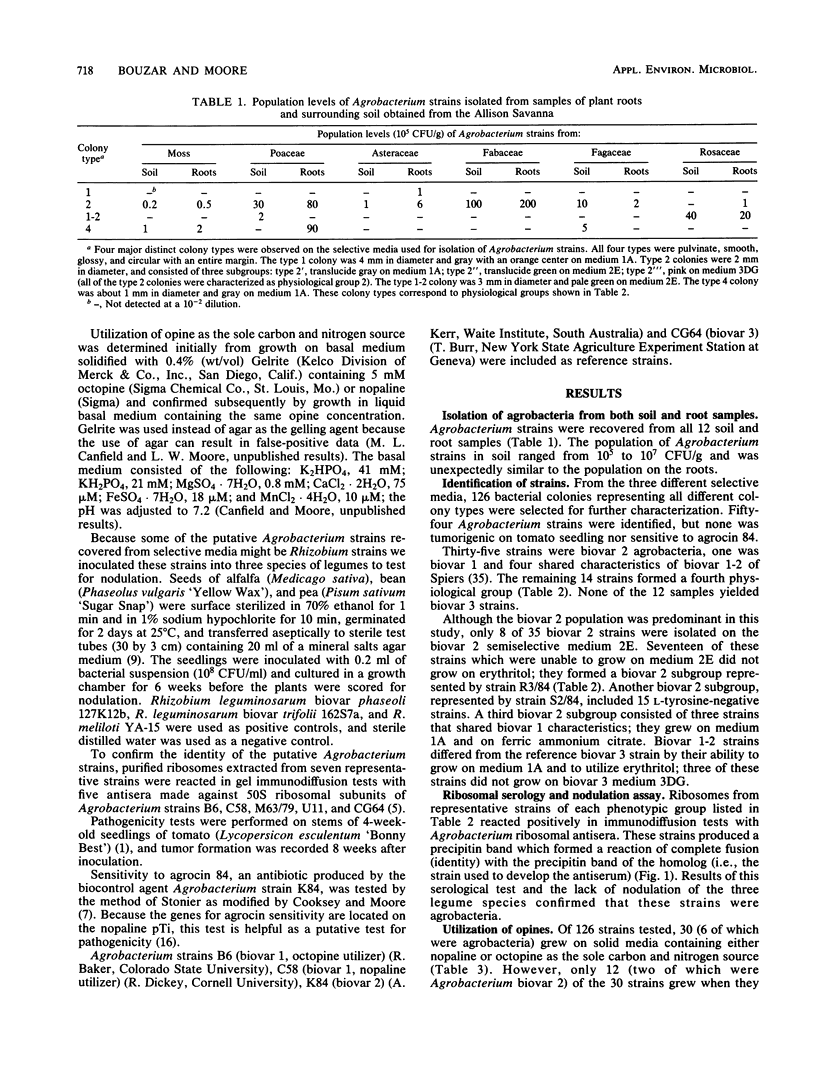
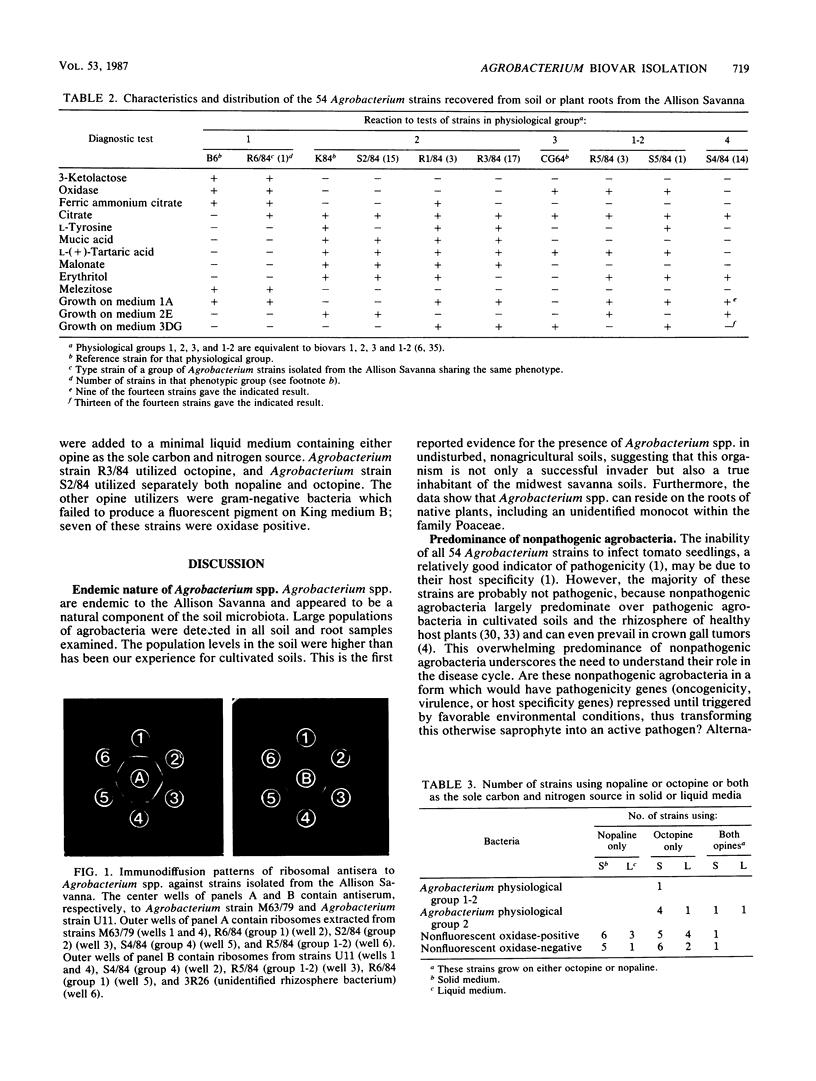
Populations of agrobacteria in excess of 105 CFU/g were recovered from 12 soil and root samples obtained from the Allison Savanna, Minn., a natural oak savanna and tallgrass prairie which has never been disturbed agriculturally. Of 126 strains picked randomly from selective media, 54 were identified as Agrobacterium spp. Biovar 2 strains predominated (35 of 54), but these strains were distributed into three phenotypically distinct subgroups. Of the remaining Agrobacterium strains, four were biovar 1-2, one was biovar 1, and none were biovar 3. The last 14 Agrobacterium strains formed a homogeneous group which differed biochemically from the hitherto reported biovars. Opine utilization (coded for by genes on the tumor-inducing plasmid in pathogenic Agrobacterium spp.) by these agrobacteria was limited to two biovar 2 strains. In contrast, 10 nonfluorescent gram-negative strains utilized either nopaline or octopine as the sole carbon and nitrogen source. There may be a need to reexamine the source and role of opines in the terrestrial environment because (i) all of these opine utilizers were isolated from an environment free of crown gall, the only known terrestrial source of opines, and (ii) 83% of the opine utilizers were not Agrobacterium spp.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A. A., Baldwin I. L., Riker A. J. Studies on Certain Physiological Characters of Phytomonas tumefaciens, Phytomonas rhizogenes and Bacillus radiobacter: Part II. J Bacteriol. 1934 Dec;28(6):597–618. doi: 10.1128/jb.28.6.597-618.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- KOVACS N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature. 1956 Sep 29;178(4535):703–703. doi: 10.1038/178703a0. [DOI] [PubMed] [Google Scholar]
- Leifson E. The Fermentation of Sodium Malonate as a Means of Differentiating Aerobacter and Escherichia. J Bacteriol. 1933 Sep;26(3):329–330. doi: 10.1128/jb.26.3.329-330.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo D. J., Nester E. W. Plasmids in avirulent strains of Agrobacterium. J Bacteriol. 1977 Jan;129(1):76–80. doi: 10.1128/jb.129.1.76-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A. L., Moore L. W., Gordon M. P., Nester E. W. Multiple genes coding for octopine-degrading enzymes in Agrobacterium. J Bacteriol. 1978 Dec;136(3):909–915. doi: 10.1128/jb.136.3.909-915.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New P. B., Kerr A. A selective medium for Agrobacterium radiobacter biotype 2. J Appl Bacteriol. 1971 Mar;34(1):233–236. doi: 10.1111/j.1365-2672.1971.tb02281.x. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Genetello C., Schell J., Schilperoort R. A., Hermans A. K., Van Montagu M., Hernalsteens J. P. Acquisition of tumour-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature. 1975 Jun 26;255(5511):742–743. doi: 10.1038/255742a0. [DOI] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]



