Abstract
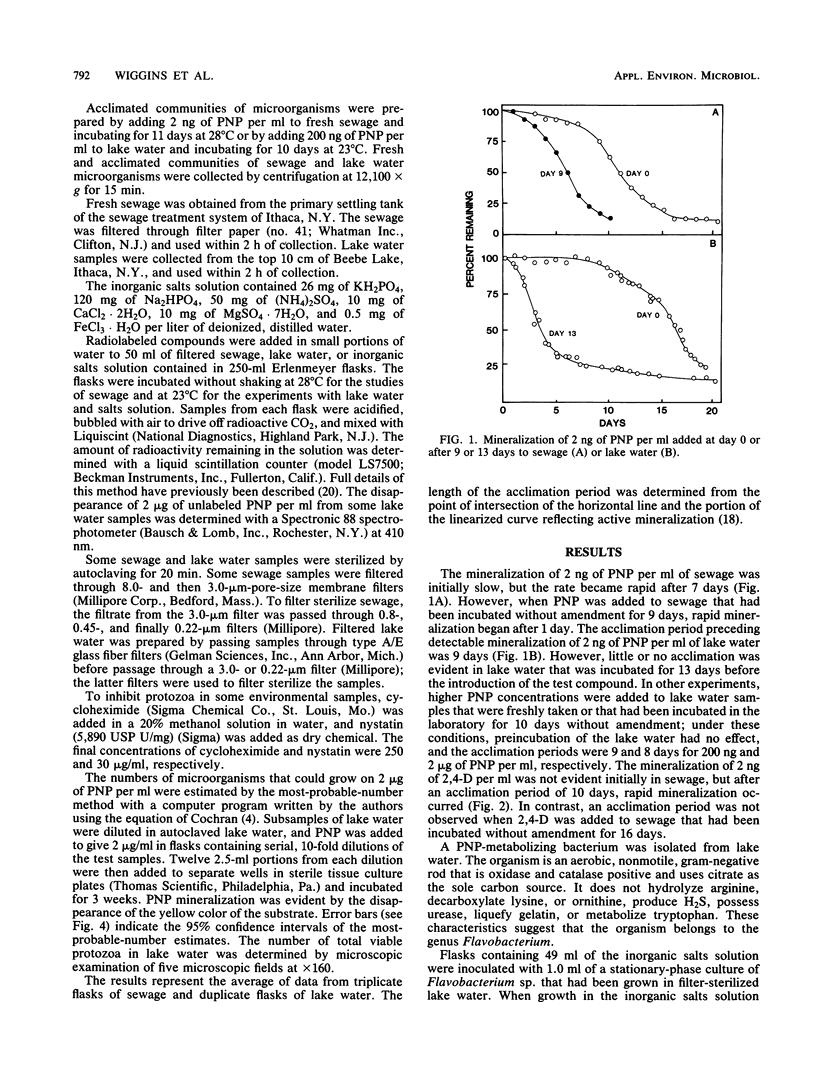
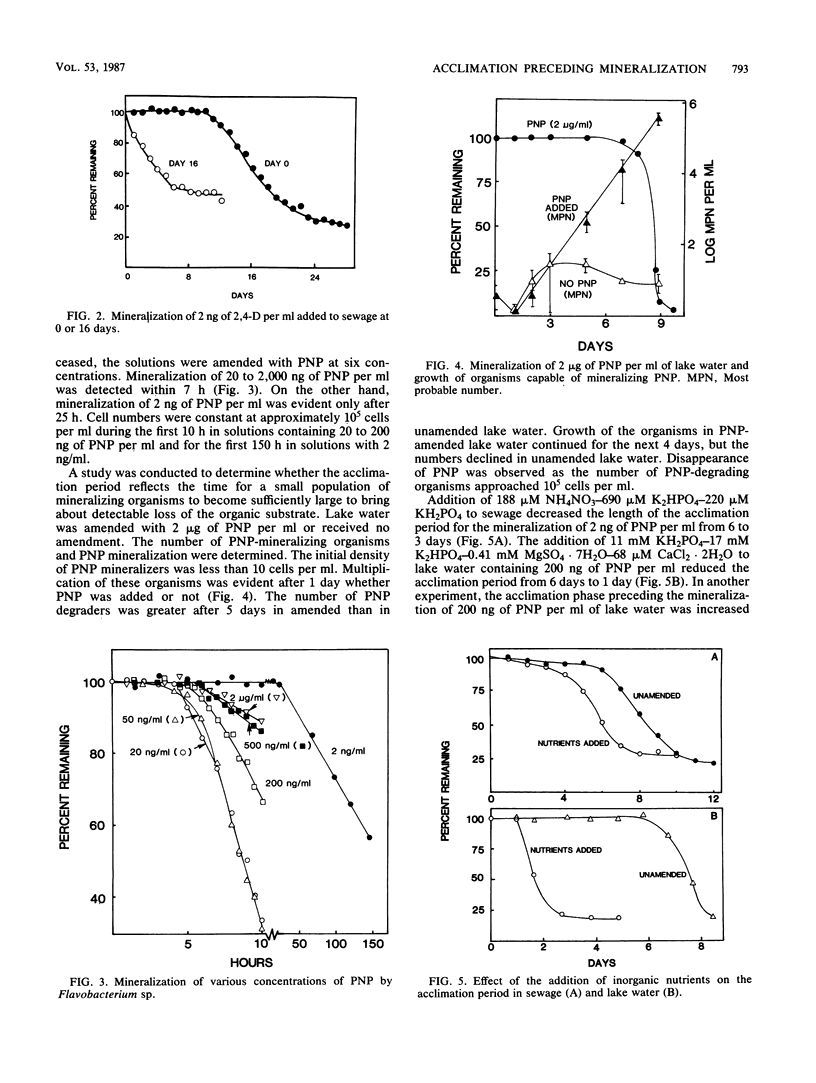
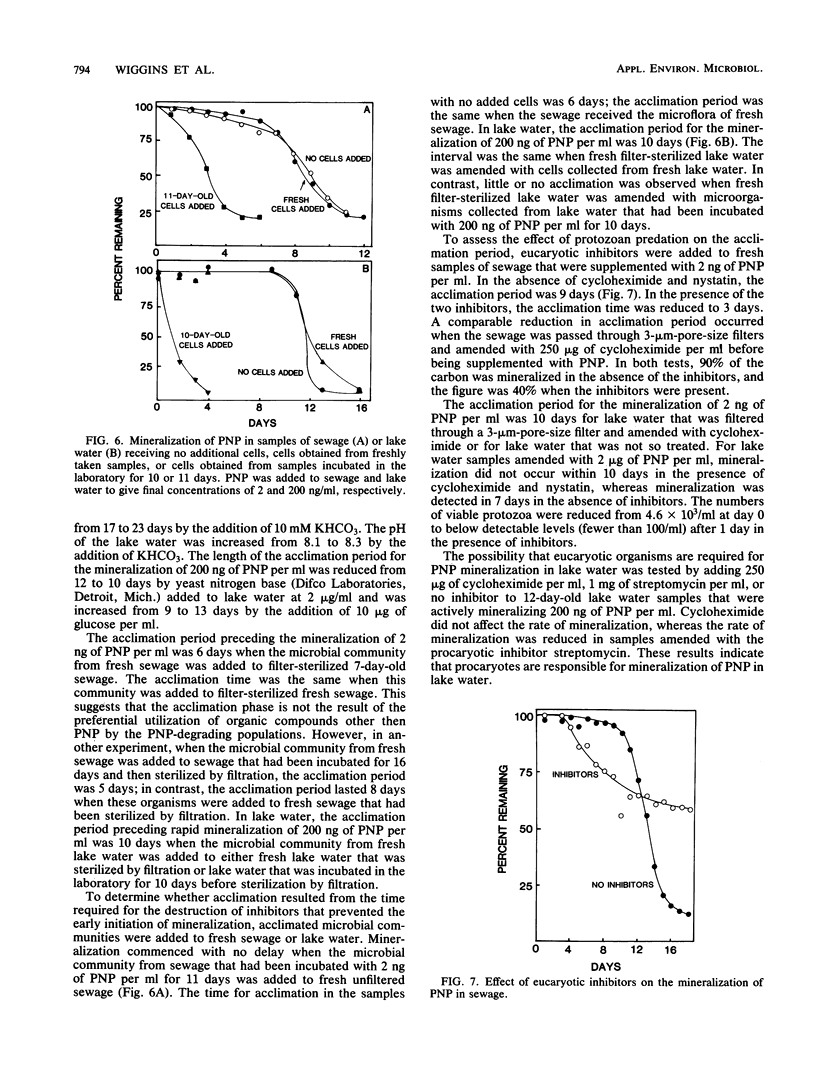
A study was conducted of possible reasons for acclimation of microbial communities to the mineralization of organic compounds in lake water and sewage. The acclimation period for the mineralization of 2 ng of p-nitrophenol (PNP) or 2,4-dichlorophenoxyacetic acid per ml of sewage was eliminated when the sewage was incubated for 9 or 16 days, respectively, with no added substrate. The acclimation period for the mineralization of 2 ng but not 200 ng or 2 micrograms of PNP per ml was eliminated when the compound was added to lake water that had been first incubated in the laboratory. Mineralization of PNP by Flavobacterium sp. was detected within 7 h at concentrations of 20 ng/ml to 2 micrograms/ml but only after 25 h at 2 ng/ml. PNP-utilizing organisms began to multiply logarithmically after 1 day in lake water amended with 2 micrograms of PNP per ml, but substrate disappearance was only detected at 8 days, at which time the numbers were approaching 10(5) cells per ml. The addition of inorganic nutrients reduced the length of the acclimation period from 6 to 3 days in sewage and from 6 days to 1 day in lake water. The prior degradation of natural organic materials in the sewage and lake water had no effect on the acclimation period for the mineralization of PNP, and naturally occurring inhibitors that might delay the mineralization were not present. The length of the acclimation phase for the mineralization of 2 ng of PNP per ml was shortened when the protozoa in sewage were suppressed by eucaryotic inhibitors, but it was unaffected or increased if the inhibitors were added to lake water.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Why microbial predators and parasites do not eliminate their prey and hosts. Annu Rev Microbiol. 1981;35:113–133. doi: 10.1146/annurev.mi.35.100181.000553. [DOI] [PubMed] [Google Scholar]
- Atlas R. M., Bartha R. Biodegradation of petroleum in seawater at low temperatures. Can J Microbiol. 1972 Dec;18(12):1851–1855. doi: 10.1139/m72-289. [DOI] [PubMed] [Google Scholar]
- COCHRAN W. G. Estimation of bacterial densities by means of the "most probable number". Biometrics. 1950 Jun;6(2):105–116. [PubMed] [Google Scholar]
- Jones S. H., Alexander M. Kinetics of mineralization of phenols in lake water. Appl Environ Microbiol. 1986 May;51(5):891–897. doi: 10.1128/aem.51.5.891-897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper J., Hanstveit A. O. Fate and effects of 4-chlorophenol and 2,4-dichlorophenol in marine plankton communities in experimental enclosures. Ecotoxicol Environ Saf. 1984 Feb;8(1):15–33. doi: 10.1016/0147-6513(84)90039-3. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Kollig H. P., Hodson R. E. Nutrient limitation and adaptation of microbial populations to chemical transformations. Appl Environ Microbiol. 1986 Mar;51(3):598–603. doi: 10.1128/aem.51.3.598-603.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWMAN A. S., WALKER R. L. Microbial decomposition of 2, 4-dichlorophenoxyacetic acid. Appl Microbiol. 1956 Jul;4(4):201–206. doi: 10.1128/am.4.4.201-206.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm N., Lindgaard-Jørgensen P., Hansen N. Biodegradation of 4-nitrophenol in standardized aquatic degradation tests. Ecotoxicol Environ Saf. 1984 Oct;8(5):451–470. doi: 10.1016/0147-6513(84)90066-6. [DOI] [PubMed] [Google Scholar]
- Pike E. B., Curds C. R. The microbial ecology of the activated sludge process. Soc Appl Bacteriol Symp Ser. 1971;1:123–147. doi: 10.1016/b978-0-12-648050-4.50012-2. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. Enzymic adaptation in bacteria: its biochemical and genetic basis. Essays Biochem. 1968;4:105–154. [PubMed] [Google Scholar]
- Schmidt E., Hellwig M., Knackmuss H. J. Degradation of chlorophenols by a defined mixed microbial community. Appl Environ Microbiol. 1983 Nov;46(5):1038–1044. doi: 10.1128/aem.46.5.1038-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Pritchard P. H., Bourquin A. W. Effects of adaptation on biodegradation rates in sediment/water cores from estuarine and freshwater environments. Appl Environ Microbiol. 1980 Oct;40(4):726–734. doi: 10.1128/aem.40.4.726-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A., Monti C. A., Pritchard P. H., Cripe C. R. Comparison of p-Nitrophenol Biodegradation in Field and Laboratory Test Systems. Appl Environ Microbiol. 1984 Nov;48(5):944–950. doi: 10.1128/aem.48.5.944-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventullo R. M., Larson R. J. Adaptation of aquatic microbial communities to quaternary ammonium compounds. Appl Environ Microbiol. 1986 Feb;51(2):356–361. doi: 10.1128/aem.51.2.356-361.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker H. H. CARBON DIOXIDE AS A FACTOR AFFECTING LAG IN BACTERIAL GROWTH. Science. 1932 Dec 23;76(1982):602–604. doi: 10.1126/science.76.1982.602. [DOI] [PubMed] [Google Scholar]



