Abstract
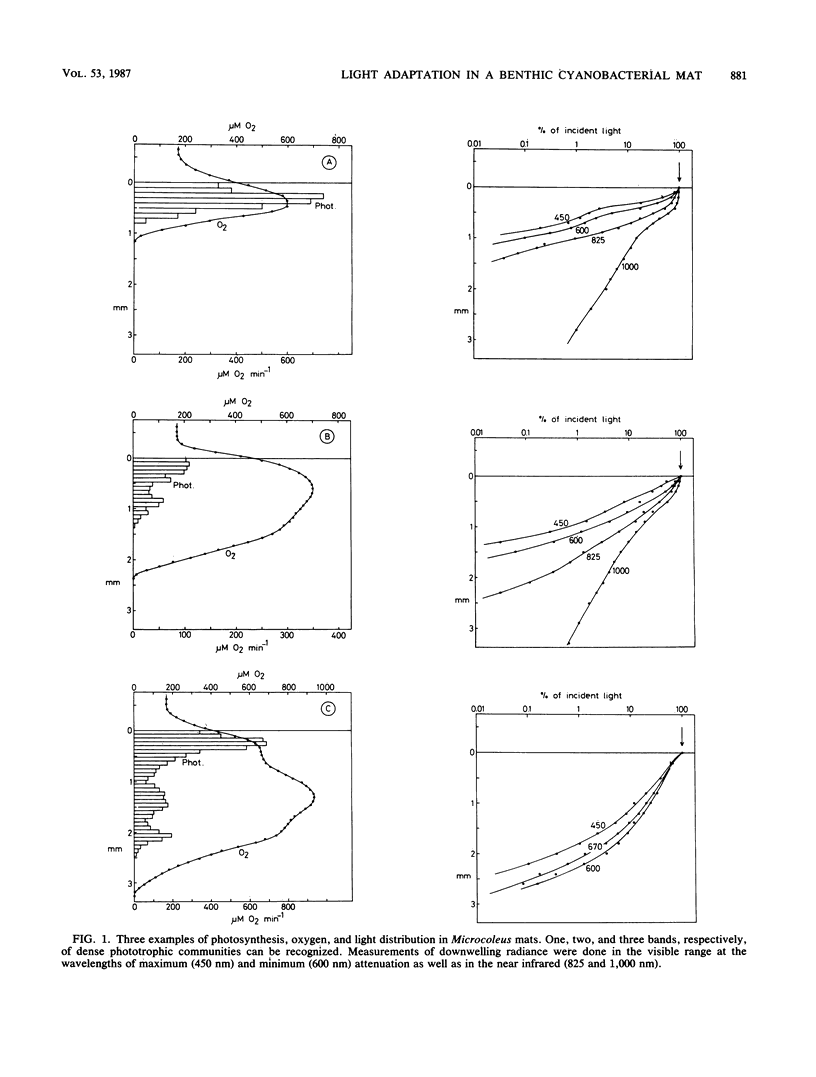
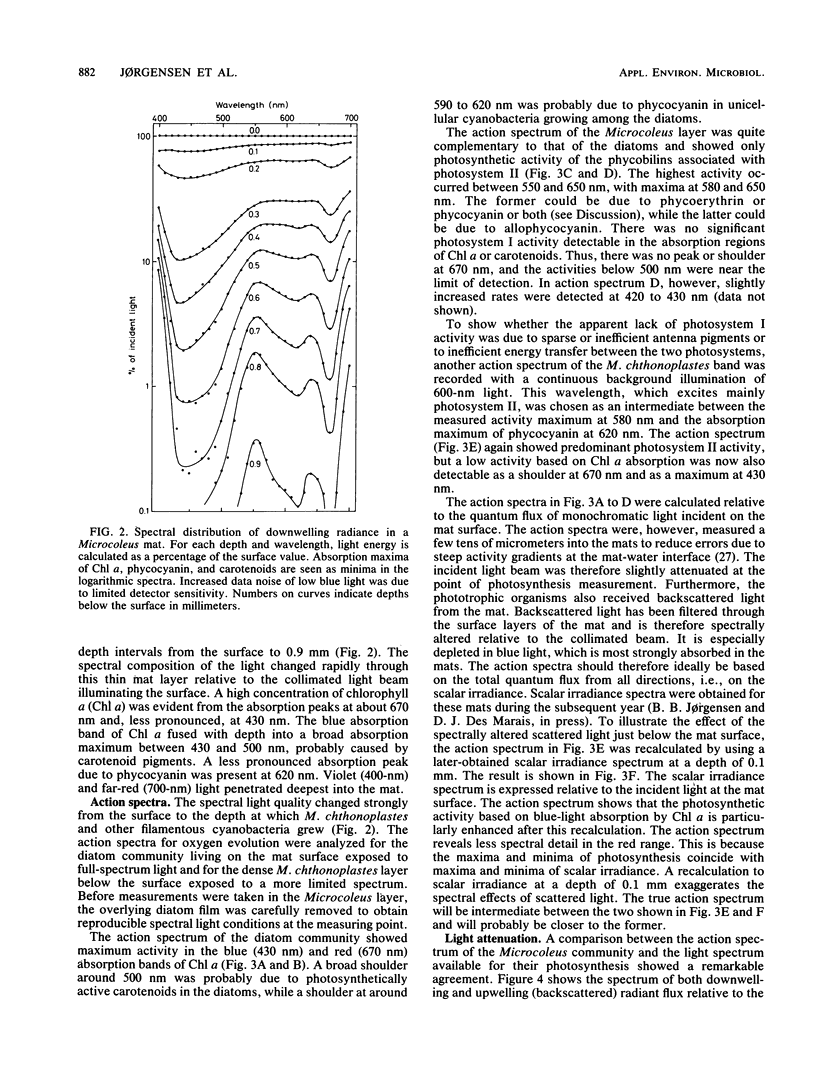
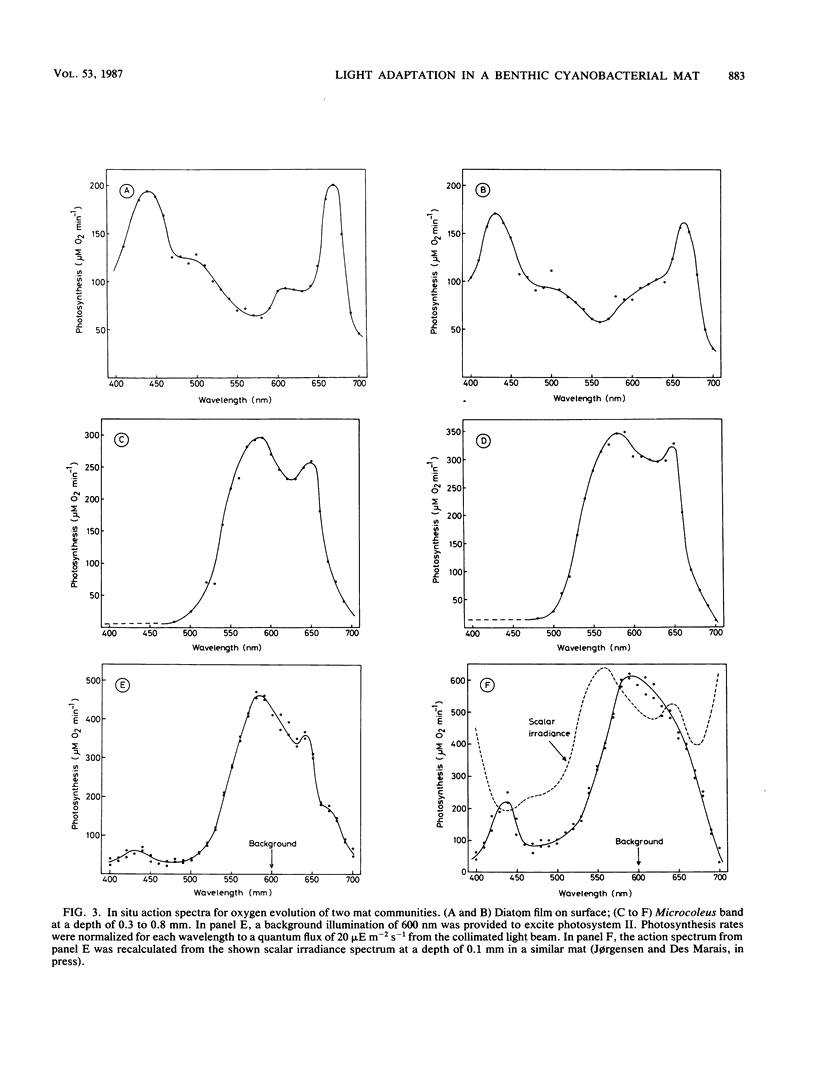
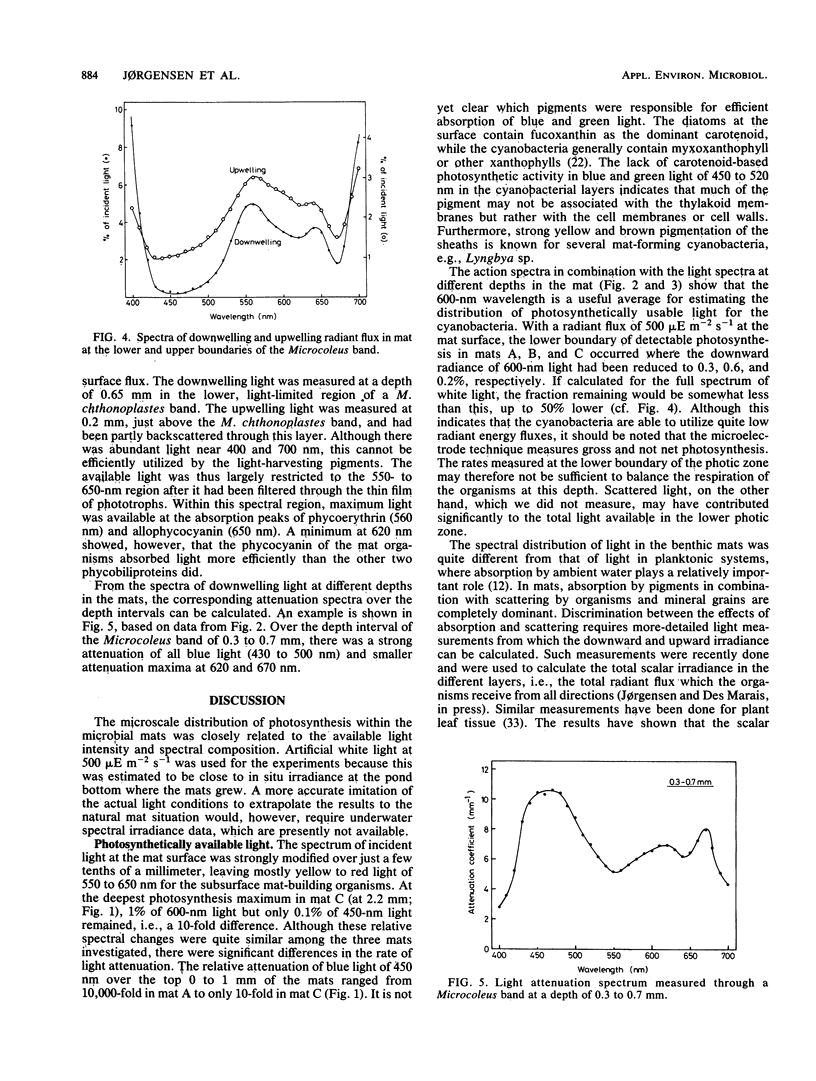
We studied adaptation to spectral light distribution in undisturbed benthic communities of cyanobacterial mats growing in hypersaline ponds at Guerrero Negro, Baja California, Mexico. Microscale measurements of oxygen photosynthesis and action spectra were performed with microelectrodes; spectral radiance was measured with fiber-optic microprobes. The spatial resolution of all measurements was 0.1 mm, and the spectral resolution was 10 to 15 nm. Light attenuation spectra showed absorption predominantly by chlorophyll a (Chl a) (430 and 670 nm), phycocyanin (620 nm), and carotenoids (440 to 500 nm). Blue light (450 nm) was attenuated 10-fold more strongly than red light (600 nm). The action spectra of the surface film of diatoms accordingly showed activity over the whole spectrum, with maxima for Chl a and carotenoids. The underlying dense Microcoleus population showed almost exclusively activity dependent upon light harvesting by phycobilins at 550 to 660 nm. Maximum activity was at 580 and 650 nm, indicating absorption by phycoerythrin and phycocyanin as well as by allophycocyanin. Very little Chl a-dependent activity could be detected in the cyanobacterial action spectrum, even with additional 600-nm light to excite photosystem II. The depth distribution of photosynthesis showed detectable activity down to a depth of 0.8 to 2.5 mm, where the downwelling radiant flux at 600 nm was reduced to 0.2 to 0.6% of the surface flux.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinks L. R. ACTION SPECTRA OF CHROMATIC TRANSIENTS AND THE EMERSON EFFECT IN MARINE ALGAE. Proc Natl Acad Sci U S A. 1960 Mar;46(3):327–333. doi: 10.1073/pnas.46.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Structure and molecular organization of the photosynthetic accessory pigments of cyanobacteria and red algae. Mol Cell Biochem. 1977 Dec 29;18(2-3):125–140. doi: 10.1007/BF00280278. [DOI] [PubMed] [Google Scholar]
- Jones L. W., Myers J. Enhancement in the Blue-Green Alga, Anacystis nidulans. Plant Physiol. 1964 Nov;39(6):938–946. doi: 10.1104/pp.39.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen B. B., Des Marais D. J. Competition for sulfide among colorless and purple sulfur bacteria in cyanobacterial mats. FEMS Microbiol Ecol. 1986;38:179–186. doi: 10.1111/j.1574-6968.1986.tb01727.x. [DOI] [PubMed] [Google Scholar]
- Lemasson C., Marsac N. T., Cohen-Bazire G. Role of allophycocyanin as light-harvesting pigment in cyanobacteria. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3130–3133. doi: 10.1073/pnas.70.11.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech N. P., Ward D. M. Oxygen Microelectrode That Is Insensitive to Medium Chemical Composition: Use in an Acid Microbial Mat Dominated by Cyanidium caldarium. Appl Environ Microbiol. 1983 Mar;45(3):755–759. doi: 10.1128/aem.45.3.755-759.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA K., BENSON A. A., CALVIN M. The absorption spectra of suspensions of living micro-organisms. Biochim Biophys Acta. 1954 Dec;15(4):461–470. doi: 10.1016/0006-3002(54)90002-5. [DOI] [PubMed] [Google Scholar]



