Abstract
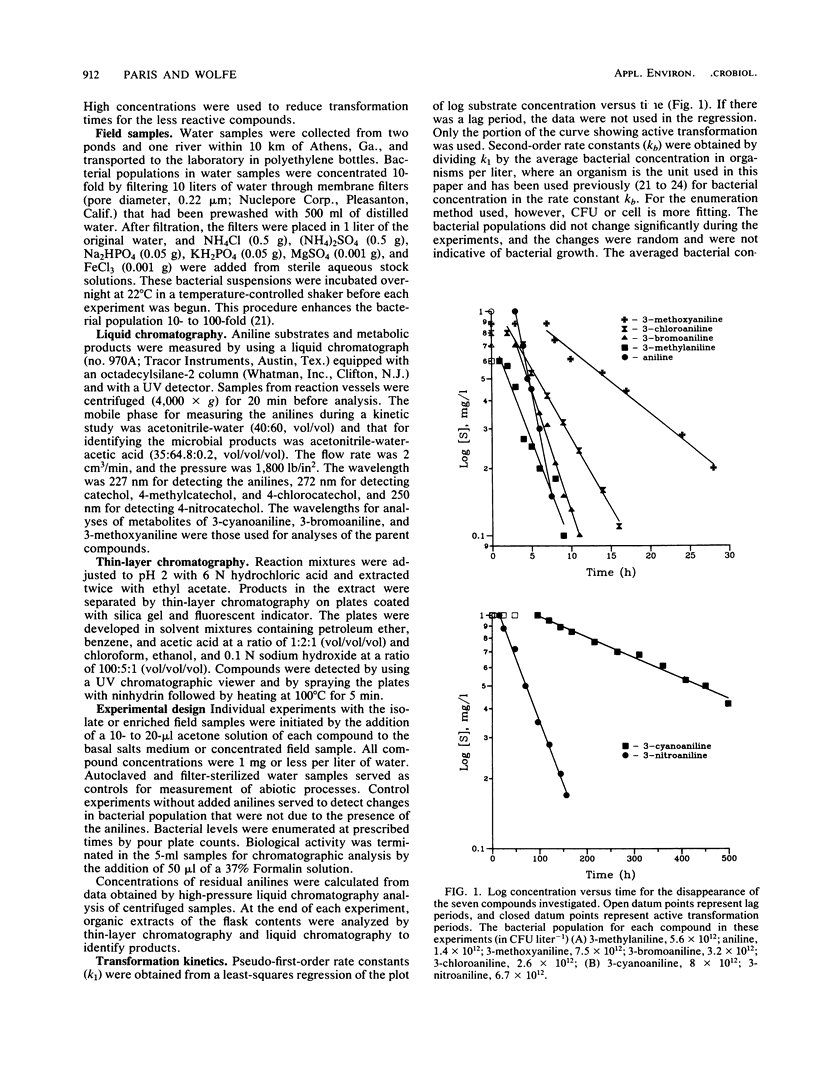
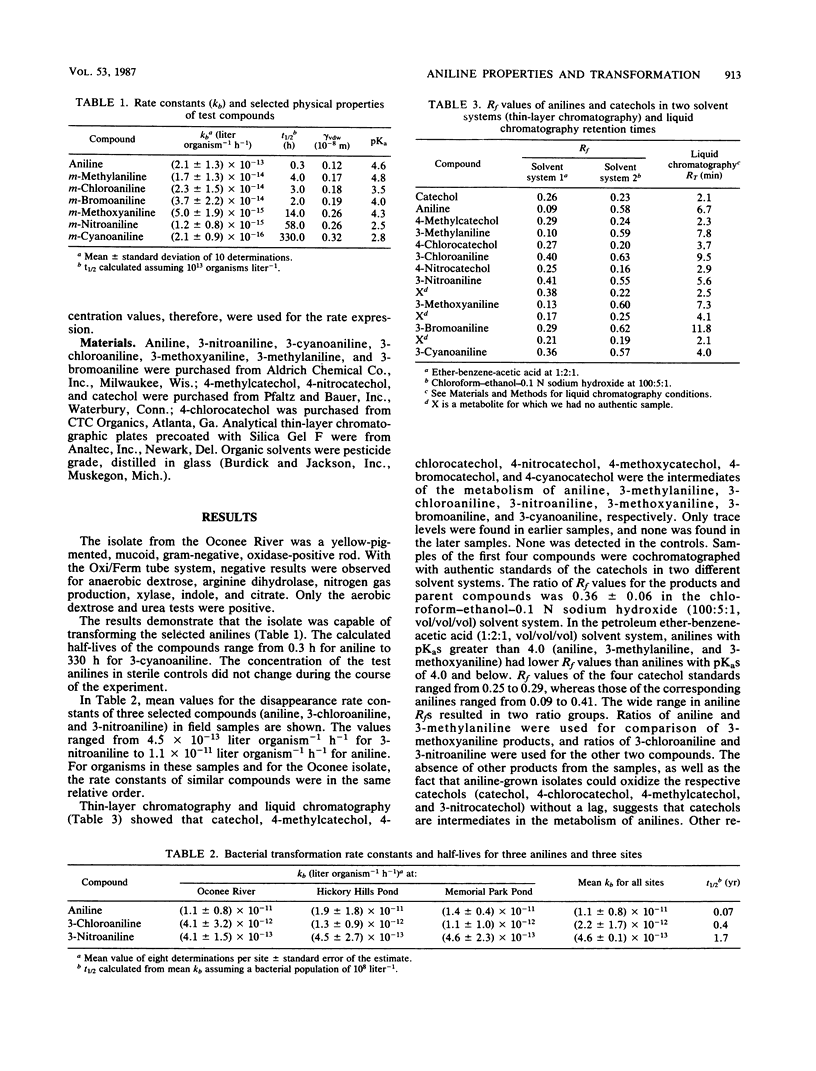
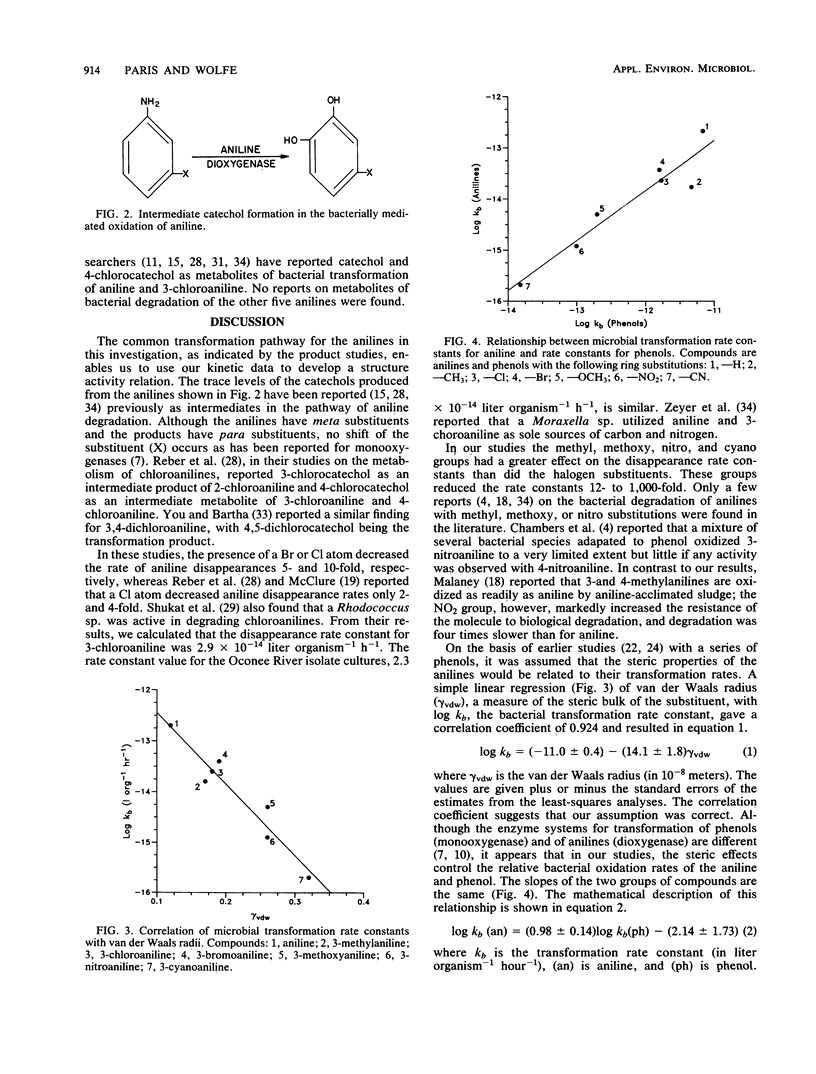
The effect of compound structure on the microbial transformation of a series of substituted anilines was investigated. For the pure-culture and environmental water samples studied, the rate of transformation of the compounds decreased in the following order: aniline greater than 3-bromoaniline greater than 3-chloroaniline greater than 3-methylaniline greater than 3-methoxyaniline greater than 3-nitroaniline greater than 3-cyanoaniline. Second-order rate constants (kb) for each compound was calculated by using bacterial and compound concentrations measured as a function of time. The rate constants correlated with steric parameters. Water samples also were used in kinetic studies with three of the compounds (aniline, 3-chloroaniline, and 3-nitroaniline) to test the relationships with mixed bacterial populations. A simple linear regression of van der Waals radius of the substituent group with log kb gave correlation coefficients (r2) of 0.924 for the river isolate and 0.99 for the mixed populations. Analyses of pure-culture and mixed-population samples by thin-layer chromatography indicate that the primary products are catechols. This finding suggests that the transformation pathway involves oxidative deamination of the anilines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T. Microbial degradation of aromatic compounds. Science. 1967 Sep 13;161(3846):1093–1097. [PubMed] [Google Scholar]
- Hayaishi O., Nozaki M. Nature and mechanisms of oxygenases. Science. 1969 Apr 25;164(3878):389–396. doi: 10.1126/science.164.3878.389. [DOI] [PubMed] [Google Scholar]
- Lyons C. D., Katz S., Bartha R. Mechanisms and pathways of aniline elimination from aquatic environments. Appl Environ Microbiol. 1984 Sep;48(3):491–496. doi: 10.1128/aem.48.3.491-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDiarmid J. E., Rose W. C., Biddle W. C., Perlman M. E., Breiner R. G., Ambrus J. L., Bardos T. J. Synthesis and properties of bis(2,2-dimethylaziridinyl)phosphinic amides: a series of new antineoplastic agents. J Med Chem. 1985 Nov;28(11):1685–1691. doi: 10.1021/jm00149a025. [DOI] [PubMed] [Google Scholar]
- McCormick N. G., Feeherry F. E., Levinson H. S. Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl Environ Microbiol. 1976 Jun;31(6):949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE W. J., FEISAL V. E. Bacterial utilization of dodecyl sulfate and dodecyl benzene sulfonate. Appl Microbiol. 1963 Jul;11:339–344. doi: 10.1128/am.11.4.339-344.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Rogers J. E. Kinetic concepts for measuring microbial rate constants: effects of nutrients on rate constants. Appl Environ Microbiol. 1986 Feb;51(2):221–225. doi: 10.1128/aem.51.2.221-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Wolfe N. L., Steen W. C., Baughman G. L. Effect of phenol molecular structure on bacterial transformation rate constants in pond and river samples. Appl Environ Microbiol. 1983 Mar;45(3):1153–1155. doi: 10.1128/aem.45.3.1153-1155.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Wolfe N. L., Steen W. C. Microbial transformation of esters of chlorinated carboxylic acids. Appl Environ Microbiol. 1984 Jan;47(1):7–11. doi: 10.1128/aem.47.1.7-11.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Wolfe N. L., Steen W. C. Structure-activity relationships in microbial transformation of phenols. Appl Environ Microbiol. 1982 Jul;44(1):153–158. doi: 10.1128/aem.44.1.153-158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Wasserfallen A., Timmis K. N. Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol. 1985 Aug;50(2):447–453. doi: 10.1128/aem.50.2.447-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



