Abstract
Streptococcus cremoris strain IL964 possessed a restriction and modification (R/M) activity which resulted in a bacteriophage efficiency of plating of 5 × 10−6. Phage sensitivity of protoplast-induced plasmid-cured derivatives indicated that two plasmids called pIL103 (5.7 kilobases) and pIL107 (15.2 kilobases) were each coding for one R/M system. Plasmid pIL103-encoded R/M was ascertained by transfer into the plasmid-free, R−/M− strain IL1403 of S. lactis, using protoplast cotransformation. This procedure failed for pIL107 because of some degree of incompatibility between pIL107 and the indicator plasmid pHV1301 used in cotransformation experiments. We also observed that plasmid pIL105 (8.7 kilobases) which showed no incidence on phage sensitivity in the parental strain IL964, mediated abortive infection in strain IL1403. In 97% of the infected cells, the phage infection was abortive, while in the remaining 3% phages were produced with a decreased burst size (50 instead of 180).
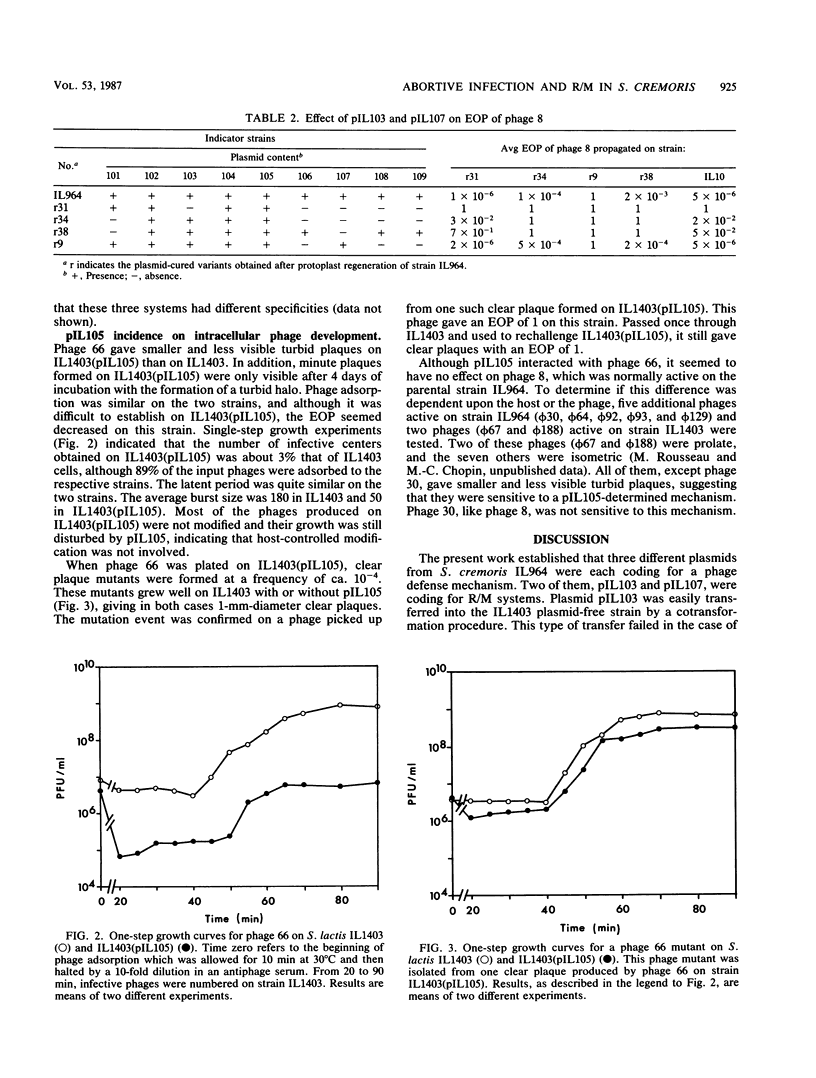
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chopin A., Chopin M. C., Moillo-Batt A., Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984 May;11(3):260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Chopin M. C., Chopin A., Rouault A., Simon D. Cloning in Streptococcus lactis of plasmid-mediated UV resistance and effect on prophage stability. Appl Environ Microbiol. 1986 Feb;51(2):233–237. doi: 10.1128/aem.51.2.233-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin M. C., Chopin A., Roux C. Definition of bacteriophage groups according to their lytic action on mesophilic lactic streptococci. Appl Environ Microbiol. 1976 Dec;32(6):741–746. doi: 10.1128/aem.32.6.741-746.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis A. W., Klaenhammer T. R. Bacteriophage Resistance Conferred on Lactic Streptococci by the Conjugative Plasmid pTR2030: Effects on Small Isometric-, Large Isometric-, and Prolate-Headed Phages. Appl Environ Microbiol. 1986 Jun;51(6):1272–1277. doi: 10.1128/aem.51.6.1272-1277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., Sanozky R. B. Conjugal transfer from Streptococcus lactis ME2 of plasmids encoding phage resistance, nisin resistance and lactose-fermenting ability: evidence for a high-frequency conjugative plasmid responsible for abortive infection of virulent bacteriophage. J Gen Microbiol. 1985 Jun;131(6):1531–1541. doi: 10.1099/00221287-131-6-1531. [DOI] [PubMed] [Google Scholar]
- Males B. M., Stocker B. A. Escherichia coli K317, formerly used to define colicin group E2, produces colicin E7, is immune to colicin E2, and carries a bacteriophage-restricting conjugative plasmid. J Bacteriol. 1980 Nov;144(2):524–531. doi: 10.1128/jb.144.2.524-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Conjugative 40-megadalton plasmid in Streptococcus lactis subsp. diacetylactis DRC3 is associated with resistance to nisin and bacteriophage. Appl Environ Microbiol. 1984 Jan;47(1):68–74. doi: 10.1128/aem.47.1.68-74.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Ozeki H. Early abortive lysis by phage BF23 in Escherichia coli K-12 carrying the colicin Ib factor. J Virol. 1968 Nov;2(11):1249–1254. doi: 10.1128/jvi.2.11.1249-1254.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Characterization of Phage-Sensitive Mutants from a Phage-Insensitive Strain of Streptococcus lactis: Evidence for a Plasmid Determinant that Prevents Phage Adsorption. Appl Environ Microbiol. 1983 Nov;46(5):1125–1133. doi: 10.1128/aem.46.5.1125-1133.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Evidence for Plasmid Linkage of Restriction and Modification in Streptococcus cremoris KH. Appl Environ Microbiol. 1981 Dec;42(6):944–950. doi: 10.1128/aem.42.6.944-950.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Phage Resistance in a Phage-Insensitive Strain of Streptococcus lactis: Temperature-Dependent Phage Development and Host-Controlled Phage Replication. Appl Environ Microbiol. 1984 May;47(5):979–985. doi: 10.1128/aem.47.5.979-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Rouault A., Chopin M. C. High-efficiency transformation of Streptococcus lactis protoplasts by plasmid DNA. Appl Environ Microbiol. 1986 Aug;52(2):394–395. doi: 10.1128/aem.52.2.394-395.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing W. D., Klaenhammer T. R. Conjugal Transfer of Bacteriophage Resistance Determinants on pTR2030 into Streptococcus cremoris Strains. Appl Environ Microbiol. 1986 Jun;51(6):1264–1271. doi: 10.1128/aem.51.6.1264-1271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenson L. R., Klaenhammer T. R. Streptococcus cremoris M12R transconjugants carrying the conjugal plasmid pTR2030 are insensitive to attack by lytic bacteriophages. Appl Environ Microbiol. 1985 Oct;50(4):851–858. doi: 10.1128/aem.50.4.851-858.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vd Pol H., Veltkamp E., Nijkamp H. J. Genetic information of the bacteriocinogenic plasmid Clo DF13 involved in the inhibition of the multiplication of double stranded DNA phages. Mol Gen Genet. 1980;178(3):535–540. doi: 10.1007/BF00337858. [DOI] [PubMed] [Google Scholar]