Abstract
Odorant information is encoded by a series of intracellular signal transduction events thought to be mediated primarily by the second messenger cAMP. We have found a subset of olfactory neurons that express the cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D (GC-D), suggesting that cGMP in these neurons also can have an important regulatory function in olfactory signaling. PDE2 and GC-D are both expressed in olfactory cilia where odorant signaling is initiated; however, only PDE2 is expressed in axons. In contrast to most other olfactory neurons, these neurons appear to project to a distinct group of glomeruli in the olfactory bulb that are similar to the subset that have been termed “necklace glomeruli.” Furthermore, this subset of neurons are unique in that they do not contain several of the previously identified components of olfactory signal transduction cascades involving cAMP and calcium, including a calcium/calmodulin-dependent PDE (PDE1C2), adenylyl cyclase III, and cAMP-specific PDE (PDE4A). Interestingly, these latter three proteins are expressed in the same neurons; however, their subcellular distribution is distinct. PDE1C2 and adenylyl cyclase III are expressed almost exclusively in the olfactory cilia whereas PDE4A is present only in the cell bodies and axons. These data strongly suggest that selective compartmentalization of different PDEs and cyclases is an important feature for the regulation of signal transduction in olfactory neurons and likely in other neurons as well. In addition, the data implies that an olfactory signal transduction pathway specifically modulated by cGMP is present in some neurons of the olfactory neuroepithelium.
The olfactory epithelium is composed almost entirely of sensory neurons that are responsible for the sense of smell. These neurons consist of cilia that extend from an apical dendrite and project into the mucosal layer of the nasal cavity where exposure to the external environment allows for the interaction of specific odorants with their respective odorant receptors. This interaction initiates transduction of the odorant signal that is thought to occur primarily through a cAMP-mediated pathway in most olfactory neurons (1). This signal is transmitted along the axons that extend from the basal portion of the neuron and project to the olfactory bulb where they terminate in distinct structures called glomeruli. Here the odorant signals are integrated and modulated followed by transmission to higher brain centers for processing. The cAMP-mediated odorant pathway is thought to consist of the seven transmembrane odorant receptors (2) coupled to adenylyl cyclase III (3, 4) via the G-protein, Golf (5). cAMP produced upon odorant binding can result in the opening of the cyclic nucleotide gated channels (6, 7) and initiation of an action potential.
Recently, the expression of an olfactory-specific guanylyl cyclase (GC-D) was identified in a distinct subset of neurons in the olfactory neuroepithelium (8). GC-D is homologous to other transmembrane guanylyl cyclase receptors where stimulation of the extracellular domain by a peptide leads to activation of the intracellular catalytic domain and production of cGMP (9), suggesting that a cGMP pathway may also be involved in olfactory signaling. The distribution pattern of GC-D mRNA throughout the neuronal layer is similar to the zonal pattern observed for the G-protein coupled odorant receptors (10, 11). No ligand has yet been identified for this receptor. In addition, unlike the hundreds of odorant receptors that have been reported to exist throughout the olfactory neuroepithelium (2), only one gene to date has been identified for GC-D in rats, although several have been identified in Caenorhabditis elegans (see ref. 12, which is in this issue of the Proceedings).
We have identified a subset of olfactory neurons that express the cGMP-stimulated phosphodiesterase (PDE2). Cyclic nucleotide PDEs (13) are responsible for the hydrolysis of the second messengers, cAMP and cGMP, and are distinguished by several features including differential regulation, hydrolytic specificities, inhibitor profiles, and sequence homology (13). The hydrolytic activity of members of the PDE2 family is increased in the presence of cGMP. PDE isoforms from two other families have also been identified in olfactory neurons. A calcium/calmodulin-dependent PDE (PDE1C2) (14, 15) and a high affinity cAMP-specific PDE (PDE4A) (16) are both expressed at high levels in a large number of olfactory neurons. PDE1C2 is thought to be responsible for the rapid turnover of cAMP seen in response to odorant exposure. A function for PDE4A at present is unclear; however, high expression in the axons suggests it may be involved in transmission of the odorant signal to the brain.
MATERIALS AND METHODS
Antisera.
PDE2 antisera were produced in rabbits and chickens (Rockland, Gilbertsville, PA) using protein purified from bovine heart and recombinant PDE2A1 protein. The chicken IgY was purified from egg yolks as described (17). PDE2 antisera was affinity purified as described (18). Glutathione-S-transferase-fusion proteins were used to make antibodies specific for the unique N terminus of PDE1C2 and the intracellular and extracellular domains of GC-D. In addition, an antisera was developed to a unique C-terminal peptide of GC-D. PDE4A antisera (19) was made as described. Adenylyl cyclase III (ACIII) antisera was purchased from Santa Cruz Biotechnology.
Immunoblot Analysis.
Olfactory neuroepithelium was homogenized in buffer containing 40 mM Tris (pH 7.5), 15 mM benzamidine, 1 μg/ml pepstatin, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Membrane and supernatant fractions were separated by a 30-min centrifugation at 10000 × g at 4°C. Equal volumes of particulate and supernatant fractions (5–50 μg), solubilized in 1% SDS, were separated by electrophoresis on an 8% SDS/polyacrylamide gel and transferred to nitrocellulose. The membranes were blocked in 5% milk protein in Tris-buffered saline (TBS)/0.1% Tween for 1 h, incubated in primary antisera for 2 h at room temperature and horseradish-peroxidase-conjugated goat anti-chicken (Jackson ImmunoResearch) secondary antibody for 1 h at room temperature. The reaction was visualized by incubation in a chemiluminescent substrate (Pierce) for 5 min and exposed to x-ray film (Kodak).
Immunohistochemistry.
C57BL/6 male or female adult mice were perfused with 4% paraformaldehyde. Nasal epithelium was removed, decalcified overnight in 0.2 M EDTA, sunk in 30% sucrose, and frozen in 50% OCT (Miles)/20% sucrose, and 20 μm sections were cut on a TissueTek II cryostat. Experiments in the olfactory bulb were done on floating tissue sections. Immunohistochemistry was performed using Vector’s horseradish-peroxidase Vectastain Elite ABC Kit according to instructions or with an anti-chicken secondary antibody coupled to horseradish-peroxidase (Jackson ImmunoResearch). The tissue was viewed and photographed on a Nikon Optiphot-2 microscope. Immunofluorescence was performed with fluorescent-tagged secondary antisera from Jackson ImmunoResearch. The double label for GC-D (or PDE4A) and PDE2 was as follows: GC-D (or PDE4A) and PDE2 antisera were applied simultaneously followed by goat anti-rabbit (GAR) conjugated to fluoroscein isothiocyanate (FITC) (1:200) and goat anti-chicken (GAC) antibody conjugated to lissamine rhodamine sulfonyl chloride (LRSC) (1:200). The triple label with PDE2/PDE4A/ACIII was as follows: reagents were applied in the following order: (i) PDE4A antisera, (ii) GAR-FITC labeled Fab fragments (1:50), (iii) unlabeled Fab fragments (1:20) to ensure that all rabbit sites are occupied, (iv) ACIII and PDE2 antisera, and (v) donkey anti-rabbit indodicarbocyanine (Cy5) (1:100) and GAC-LRSC. The double label for PDE1C2 and ACIII or PDE1C2 and PDE4A was as follows: (i) PDE1C2, (ii) GAR-FITC-labeled Fab fragments, (iii) unlabeled Fab fragments, (iv) ACIII or PDE4A, and (v) DAR-LRSC. The control for those experiments that included two antisera from the same host (rabbit) included omission of the second primary antisera only to verify that the second labeled secondary antisera was not binding to the first primary antisera. Labeled anti-rabbit Fab fragments were used in these experiments to prevent subsequently applied rabbit antisera from binding to the free arms of whole anti-rabbit IgG secondary antisera. Images were collected so that no pixels were saturated based on histogram analysis using a Bio-Rad MRC600 confocal microscope with comos 6.01 software and printed on a Tektronix dye-sublimation printer.
Acetylcholinesterase (AChE) Assay.
After immunohistochemisty was completed, the olfactory bulb sections were floated into AChE substrate mix as described (20).
In Situ Hybridization.
Antisense and sense nonradioactive RNA probes were labeled with digoxigenin-UTP (Boehringer Mannheim) from the linearized whole PDE2 clone and nucleotides 1–1638 of GC-D in pBluescript (Stratagene). The RNA probes were hydrolyzed under alkaline conditions to yield probes 200–500 bp long. Olfactory epithelial tissue sections were collected as described for immunohistochemistry. Tissue pretreatment included digestion by 1 μg/ml proteinase K for 10 min at 37°C followed by acetylation in 0.1 M triethanolamine and 0.25% acetic anhydride. Tissue was hybridized in buffer containing ≈1 μg/ml labeled probe and incubated overnight at 52°C. After slides were washed they were treated with alkaline phosphatase labeled anti-digoxigenin antisera and the reaction was visualized with the substrate nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate for several hours.
RESULTS
Localization of PDE2 in a Subset of Olfactory Neurons.
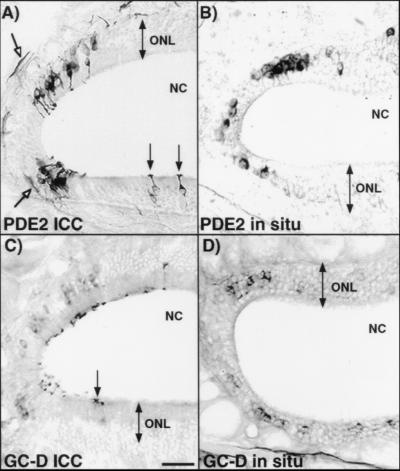
Examination of the olfactory neuronal layer for the presence of PDE2 by immunohistochemistry (Fig. 1A) and in situ hybridization (Fig. 1B) revealed a subset of olfactory neurons located in clustered groups throughout the adult mouse olfactory neuroepithelium. The most abundant groups of PDE2 containing neurons were found in areas adjacent to the olfactory bulb in caudal regions of the nasal epithelium (see Fig. 7A) and were usually localized in the recesses of olfactory turbinates (Figs. 1A and 5B). Occasional isolated neurons were also found scattered throughout the olfactory neuronal layer. Cells that express PDE2 have cilia that extend into the mucosal area (solid arrows in Figs. 1A and 3B) and axons forming bundles that project toward the olfactory bulb (open arrows in Figs. 1A and 7A) indicating that these cells are neurons. Localization of the PDE2 protein was verified by in situ hybridization studies using antisense digoxigenin-labeled riboprobes (Fig. 1B).
Figure 1.
PDE2 and GC-D are expressed in a subset of olfactory neurons. (A and C) Immunohistochemical localization of PDE2 and GC-D, respectively. (B and D) In situ hybridization localization of PDE2 and GC-D, respectively. The localization pattern of both proteins and mRNAs are similar. Open arrows denote PDE2 protein in axon fibers. Closed arrows point to cilia containing PDE2 or GC-D proteins extending from labeled neurons. NC, nasal cavity; ONL, olfactory neuron layer. (Bar = 50 μm.)
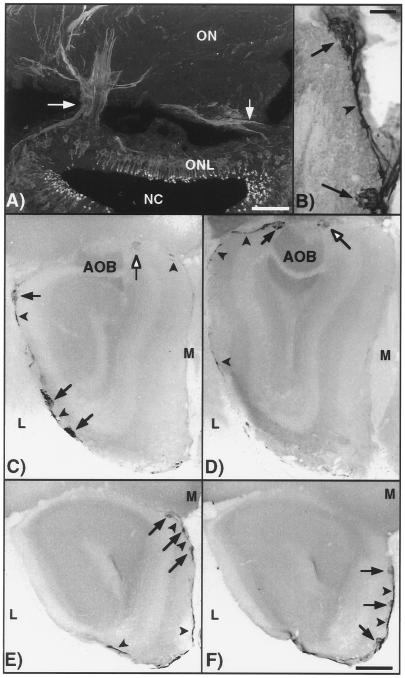
Figure 7.
Olfactory neurons that express PDE2 and GC-D project to a specific group of glomeruli in the olfactory bulb. (A) Projection of confocal images showing presence of PDE2 in axons forming bundles (arrows) and joining the olfactory nerve. (Bar = 50 μm.) (B) Two glomeruli (arrows) expressing PDE2 joined by labeled nerve fibers (arrowhead). (Bar = 200 μm.) (C–F) Serial sections (anterior-posterior) of the olfactory bulb demonstrating the pattern of glomeruli immunolabeled with PDE2 antisera (arrows). The modified glomerular complex is indicated by open arrows. Labeled nerve fibers are indicated by arrowheads. The labeled glomeruli “encircle” the caudal portion of the olfactory bulb. ONL, olfactory neuron layer; NC, nasal cavity; ON, olfactory nerve; M, medial; L, lateral. (Bar = 1 mm.)
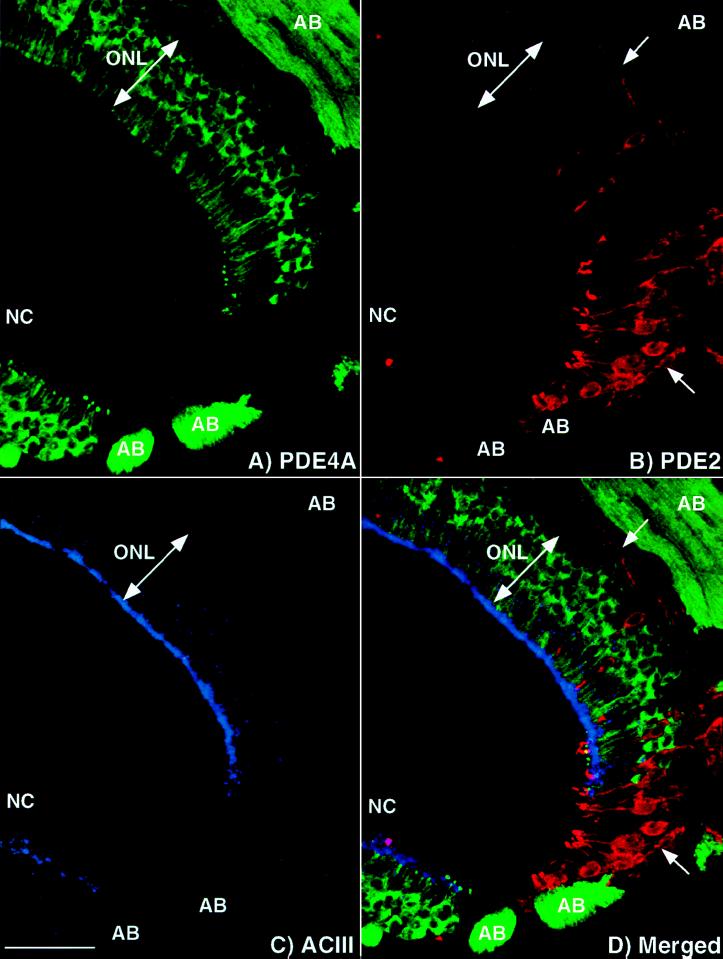
Figure 5.
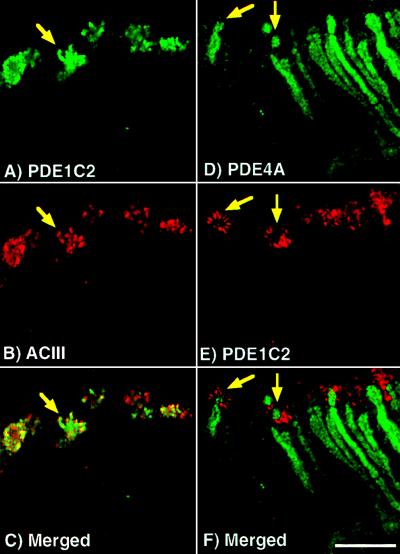
PDE2 and GC-D are found in a subset of olfactory neurons distinct from those neurons expressing ACIII and PDE4A. Projection of a triple-labeled image demonstrating a unique subset of neurons containing PDE2: (A) PDE4A, (B) PDE2, (C) ACIII, and (D) merged image of A–C. None of these proteins is colocalized with another, indicated by no overlap of the signal upon merging of the images. ONL, olfactory neuron layer; AB, axon bundle; NC, nasal cavity. Arrow denotes axons expressing PDE2. (Bar = 50 μm.)
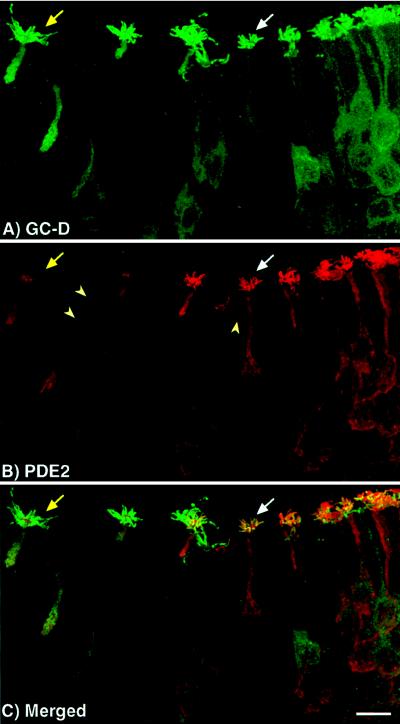
Figure 3.
Colocalization of PDE2 and GC-D in olfactory neurons. Confocal microscopy was used to determine that PDE2 and GC-D are found in the same neurons. (A and B) Unmerged image of olfactory neurons labeled with the GC-D and PDE2 antisera, respectively. (C) The merged image showing colocalization of the two proteins. PDE2 and GC-D are found in the same neurons. Some neurons, however, appear to express a lower level of PDE2 than GC-D (yellow arrow) in their cilia as compared with most PDE2/GC-D containing neurons (white arrow). Another unidentified cell type found throughout the neuroepithelium is also labeled by PDE2 antisera (arrowheads). (Bar = 10 μm.)
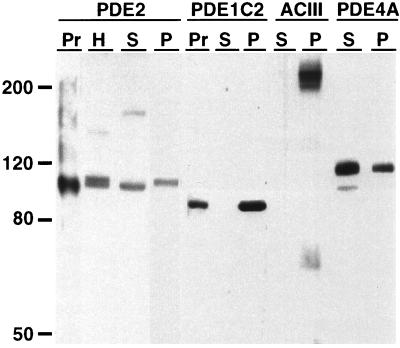
Antisera developed against PDE2 were tested for specificity by immunoblot analysis. Affinity purified antisera recognize 1 nanogram or less of Sf9 cell expressed bovine PDE2A1 (21). The major protein recognized in olfactory homogenates appears to be a doublet that migrates at the same molecular weight as the PDE2 protein (Fig. 2). The smaller protein of the doublet corresponds to the band seen in the supernatant, whereas the larger band represents the protein present in pellet fractions. This suggests that there may be two forms of PDE2 present in olfactory tissue. A population of unidentified cells is present in the olfactory neuroepithelium (arrowheads Fig. 3B) that might express a different isoform of PDE2 than the one expressed in the olfactory neurons.
Figure 2.
Immunoblot analysis of PDE2, PDE1C2, ACIII, and PDE4A antisera in olfactory homogenates. Pellet (lanes P) and supernatant (lanes S) fractions of olfactory tissue were probed with each antisera to determine if the protein is associated with soluble or particulate fractions and to verify specificity of the antisera. Affinity purified anti-PDE2 (1:100) recognizes a doublet in olfactory homogenates (lane H). The smaller protein is present in supernatant fractions while the larger protein is present in the pellet fractions. Anti-ACIII (1:5000) and affinity purified anti-PDE1C2 (1:1000) are found entirely in pellet fractions while most of the PDE4A (1:5000) is found in supernatant fractions with some immunoreactivity in pellet fractions. Pr, expressed protein.
Localization of GC-D in a Subset of Olfactory Neurons.
An olfactory-specific guanylyl cyclase receptor (GC-D) was recently found to be expressed in a subset of olfactory neurons by in situ hybridization. Antisera developed against GC-D were used for immunolabeling to examine the cellular distribution of the protein and to determine if PDE2 and GC-D were found in the same subset of neurons. The expression pattern of GC-D protein in the olfactory epithelium is consistent with that previously observed (Fig. 1 C and D). GC-D appears to be expressed at highest concentrations in the olfactory cilia (arrows in Figs. 1C and 3A), which would be expected if this protein were acting as a receptor for odorants or other molecules in the nasal cavity. Some signal was seen in cell bodies and little or no signal was seen in the axons at this level of detection. Localization in alternating serial sections suggested that GC-D had a similar distribution pattern as that demonstrated for PDE2.
Colocalization of PDE2 and GC-D.
It was important to determine if both PDE2 and GC-D were present in the same neurons because activation of GC-D would likely result in an increase of cGMP that could consequently stimulate the activity of PDE2. Immunofluorescent labeling and confocal microscopy were used to answer this question. As seen in Fig. 3 neurons containing PDE2 (Fig. 3B) also expressed GC-D (Fig. 3A) as indicated by signals for both proteins appearing in the same cell when the images were merged (Fig. 3C). Most neurons contained high levels of both PDE2 and GC-D in the olfactory cilia as indicated by the white arrows. There are, however, some neurons that appeared to express lower levels of PDE2 as compared with GC-D (yellow arrows), suggesting that the levels of protein expression in these cells may be differentially controlled. Although both PDE2 and GC-D were found in the same cells, their subcellular distribution was not identical. Both were found in areas of signal initiation (cilia); however, PDE2 was also found in areas of signal transmission (axons).
PDE2 and GC-D Are Expressed in a Subset of Neurons Distinct from Those That Express PDE1C2, PDE4A, and ACIII.
PDE1C2 (15), PDE4A (16), and ACIII (4) are components of the olfactory signal transduction cascades regulated by cAMP and calcium. These proteins are expressed at high levels in olfactory neurons and are presumed to be found in the large majority of neurons throughout the olfactory epithelium. Therefore, we wanted to determine if the cells that contain PDE2 and GC-D are a subset of this larger population of neurons or if they might represent a unique group that utilize a different signal transduction pathway.
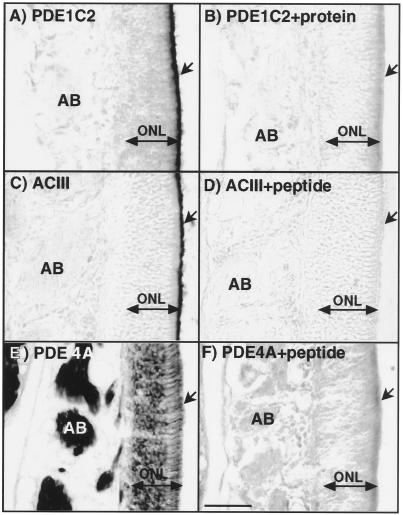
Individual immunolocalization studies of PDE1C2, PDE4A, and ACIII are shown in Fig. 4. We developed an antisera specific for the PDE1C2 isoform of the PDE1 family. This antisera results in labeling of the cilia only with very little signal in cell bodies (Fig. 4A). This differs somewhat from previous data which suggested that a PDE1 was found throughout the olfactory neuron (14); however, the antisera used in that study was not isoform specific and is known to recognize calmodulin alone as well as other calmodulin-dependent enzymes. ACIII localization in olfactory neurons has been well established (4) and is known to be localized exclusively to cilia as shown in Fig. 4C. PDE4A expression has also been recently identified in olfactory neurons (16). As reported, we have shown using an antisera that recognizes all PDE4A splice variants, that its localization was unique. No protein was found in the cilia but it was distributed throughout the rest of the neuron from dendritic knobs to axons (Fig. 4E). Immunoblot analysis was performed to demonstrate specificity of the antisera (Fig. 2). In particulate fractions of olfactory homogenates anti-ACIII detected a major band at 200 kDa, the size determined for the glycosylated form of the enzyme (4). PDE1C2 was also expressed in the particulate fractions, which suggests that it may be associated with ciliary membranes. PDE4A was detected most abundantly in the supernatant fractions with a small amount present in the particulate fraction. All antisera were competed by the appropriate peptide or protein in both immunohistochemistry experiments (Fig. 4 B, D, and F) and immunoblot analysis.
Figure 4.
Localization of PDE1C2, ACIII, and PDE4A in olfactory neuronal tissue. (A, C, and E) Immunohistochemical localization of PDE1C2, ACIII, and PDE4A in olfactory neurons, respectively. (B, D, and F) Protein and peptide competition controls of PDE1C2, ACIII, and PDE4A, respectively. The localization of PDE1C2 and ACIII is found exclusively in olfactory cilia. PDE4A is completely absent from cilia but found throughout the rest of the neuron with high expression levels in axons. Competition controls show complete block of antisera binding verifying the specificity of the reactions. Arrow, cilia layer; AB, axon bundles; ONL, olfactory neuronal layer. (Bar = 50 μm.)
We discovered using triple label immunofluorescence that PDE2, and therefore GC-D, was found in a subset of cells separate from the large majority of neurons expressing PDE1C2, PDE4A, and ACIII. This is clearly shown in Fig. 5, where olfactory tissue was labeled simultaneously with antisera against PDE4A (Fig. 5A), PDE2 (Fig. 5B), and ACIII (Fig. 5C). The merged image (Fig. 5D) shows that each antisera labels a distinct portion of the olfactory epithelium with no overlap of signal. Similar results were seen when PDE1C2 antisera was substituted for ACIII antisera. No neurons have been identified that contain both PDE4A and PDE2, suggesting that this subset of neurons use unique mechanisms of olfactory signaling.
PDE1C2, PDE4A, and ACIII Are Expressed in the Same Olfactory Neurons.
When observed at a higher magnification. PDE1C2, PDE4A, and ACIII appear to be found in the same neurons; however, their subcellular distribution is distinct. To verify that these proteins were expressed in the same neurons, tissue sections were double labeled with PDE1C2 and ACIII (Fig. 6 A–C) or PDE1C2 and PDE4A (Fig. 6 D–F) antisera. Unmerged images of PDE1C2 and ACIII immunostaining are shown in Fig. 6 A and B, respectively. When the images were merged (Fig. 6C), it appeared as if both proteins were found in the same olfactory neurons with the majority of signal for both proteins being expressed in the cilia. For example, the arrow (Fig. 6 A–C) points to one tuft of olfactory cilia that extends from a single neuron. PDE4A and PDE1C2 were also found in the same neurons; however, they were not expressed in the same subcellular compartments. The yellow arrows (Fig. 6 D–F) point to two neurons where cilia expressing PDE1C2 appear to emerge from neurons containing PDE4A. Although it is difficult to determine colocalization in the majority of neurons expressing these proteins, these data suggest that at least a subset of olfactory neurons that express PDE4A also express PDE1C2 and ACIII in their cilia.
Figure 6.
PDE1C2, ACIII, and PDE4A are expressed in the same neurons; however, their subcellular distribution is distinct. (A and B) Unmerged image of PDE1C2 and ACIII, respectively. (C) Merged image of PDE1C2 and ACIII. PDE1C2 and ACIII appear to be colocalized in the same cilia. The arrows point to a tuft of cilia emerging from a single neuron. (D and E) Unmerged image of PDE4A and PDE1C2, respectively. (F) Merged image of PDE4A and PDE1C2. Cilia expressing PDE1C2 are seen emerging from neurons expressing PDE4A (arrows), suggesting that although these proteins are found in the same neurons their subcellular distribution is distinct. (Bar = 10 μm.)
The Subset of Olfactory Neurons That Express PDE2 and GC-D Project to “Necklace Glomeruli.
” Axons originating from olfactory neurons coalesce into bundles that form the olfactory nerve and project to the olfactory bulb where they terminate in distinct structures called glomeruli. Previous studies have shown that all olfactory neurons expressing the same odorant receptor messenger RNA converge on one or a few glomeruli (22, 23). Therefore, we believed that a determination of where the subset of neurons containing PDE2 and GC-D project might suggest a function for this group of neurons. Conveniently, PDE2 is present in the axons of this unique subset of olfactory neurons, and therefore it seemed possible that the glomeruli in which these cells terminate could be determined by immunolabeling. In Fig. 7A, axons from olfactory neurons are clearly seen forming bundles (arrows) and entering the nerve layer adjacent to the olfactory bulb. PDE2 signal is detected in fibers of the olfactory nerve layer in each olfactory bulb serial section progressing posteriorly to a distinct group of about 12 glomeruli in the caudal region of both halves of the olfactory bulb (Fig. 7 C–F). This pattern of staining is consistent with axon terminals from neurons in the olfactory epithelium synapsing in glomeruli of the olfactory bulb. Further experimentation is needed, however, to unequivocally verify that all of the signal seen in these glomeruli actually originates in the olfactory neuroepithelium and that all of the axons originating from the PDE2-containing olfactory neurons terminate here.
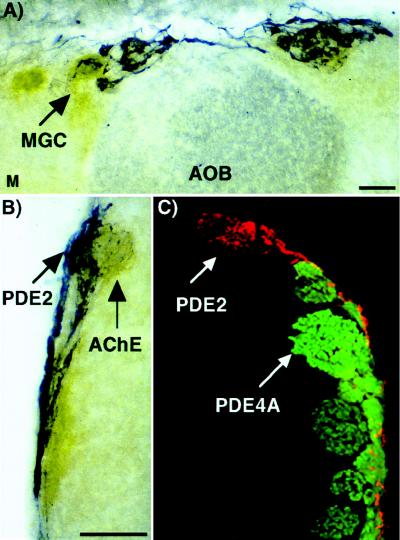
The gross morphology of these PDE2 labeled glomeruli resemble the unique structures called “atypical glomeruli” previously identified by their location in the glomerular layer and the presence of high AChE activity (24). In addition, placental antigen (PAX), which is associated with the conversion of androgens to estrogen, has been shown to be localized to a distinct subset of glomeruli adjacent to the those containing high AChE activity forming “glomerular complexes” (20). These glomerular complexes, interconnected by nerve fibers, resemble a beaded necklace encircling the caudal region of the olfactory bulb, and therefore, have been termed necklace glomeruli. Two of the PDE2 labeled glomeruli connected by labeled fibers are shown in Fig. 7B. To verify that the PDE2 labeled glomeruli are associated with the necklace glomerular complexes, we colabeled olfactory bulb sections with PDE2 antisera and by an AChE histochemical activity assay. The presence of PDE2 immunoreactivity (Fig. 8 A and B, blue) in glomeruli adjacent to those containing high AChE activity (brown) in olfactory bulb tissue sections strongly suggest that the glomeruli containing PDE2 are components of these necklace glomerular complexes. PAX is also found in a subset of olfactory neurons (25) similar to those containing PDE2 and GC-D. Whether the subset of neurons and corresponding glomeruli that express PDE2 and GC-D are identical to the subset containing PAX or are separate components of the same glomerular complex will require double labeling experiments with the PAX antisera.
Figure 8.
PDE2 is found in glomerular complexes associated with high AChE activity (atypical glomeruli) but not to the same glomeruli that express PDE4A. (A and B) Olfactory bulb tissue sections were labeled with PDE2 antisera (blue) followed by an AChE activity assay (brown). The PDE2 signal is always associated with an area high in AChE activity. This is indicative of atypical glomeruli. (C) Olfactory bulb tissue was double labeled with PDE2 (red) and PDE4A (green) antisera. The signal appears in different glomeruli. MGC, modified glomerular complex; AOB, accessory olfactory bulb; M, medial. (Bar = 200 μm.)
One complex in the “necklace” called the modified glomerular complex (Fig. 8A), located at the medial border between the accessory olfactory bulb and the main olfactory bulb, has been suggested to be associated with suckling behavior, a pheromone induced response, in rat pups (26, 27). These data might suggest that the function of this group of neurons and glomeruli is related to behavioral responses induced by hormones or pheromones, rather than a response to specific odorants.
Finally, the data also suggest that axons expressing PDE4A project to glomeruli different from those containing PDE2 (Fig. 8C). Therefore, not only do the olfactory neurons containing PDE2 and GC-D lack expression of PDE4A, ACIII, and PDE1C2, but their axons also project to different glomeruli, suggesting that this group of neurons and glomeruli form a distinct functional unit whose function might be modulated specifically by cGMP.
DISCUSSION
Expression of PDE2 and GC-D in a Subset of Olfactory Neurons.
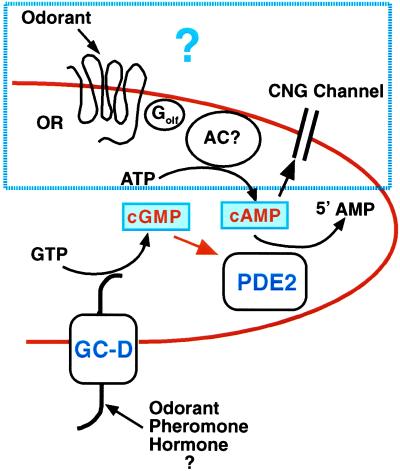
We have identified a unique subset of olfactory neurons in the nasal epithelium by immunohistochemical and in situ hybridization localization of the cGMP-stimulated PDE2 and the olfactory-specific GC-D. PDE2 and GC-D are colocalized in distinct groups of olfactory neurons widespread throughout the olfactory epithelium. What makes this subset of olfactory neurons unique is the fact that they do not contain the more ubiquitously localized PDE1C2, PDE4A, or ACIII. In addition, these neurons project to a distinct subset of glomeruli in the olfactory bulb. These data suggest that these neurons utilize a signal transduction pathway that is different from that thought to be present in most other olfactory neurons and that cGMP may be an important second messenger in this subset of neurons. Both PDE2 and GC-D are important components of cGMP signal transduction pathways. GC-D is homologous to the natriuretic membrane guanylyl cyclase receptors, as well as the heat-stable enterotoxin receptors, where stimulation of the extracellular domain by a peptide hormone leads to activation of the intracellular catalytic domain and production of cGMP. PDE2 is composed of an allosteric binding site for cGMP and a catalytic site that will hydrolyze both the second messengers, cAMP and cGMP (28). The catalytic activity of PDE2 is stimulated by submicromolar quantities of cGMP; therefore, it is quite likely that in this subset of olfactory neurons activation of GC-D leads to stimulation of PDE2 and regulation of a cyclic nucleotide mediated cell function as indicated in the model described in Fig. 9. To date, however, no ligand has yet been identified for the GC-D receptor. Subcellularly, PDE2 is found throughout the cell from cilia to axon. GC-D is found most abundantly in the cilia with relatively less expression in cell bodies and little or no protein detectable in axons.
Figure 9.
Schematic model of olfactory cilia containing PDE2 and GC-D. GC-D is a transmembrane guanylyl cyclase receptor. Activation of this receptor by its ligand (unknown at present) could result in an increase of cGMP that could activate PDE2 and stimulate hydrolysis of cAMP as well as cGMP. In most neurons odorants are thought to stimulate adenylyl cyclase activity by binding to the G-protein (Golf)-coupled seven transmembrane odorant receptor (OR). The cyclic nucleotide gated channel (CNG) is then opened by cAMP, resulting in transmission of a signal along the axon to the olfactory bulb. We have shown that there is no detectable expression of ACIII or PDE1C2 in these neurons; however, it is not known at present if there is an OR, Golf, or a CNG channel or which cell function in this population of neurons is under control of a cAMP-mediated pathway.
Roles of PDE2 and GC-D in Olfactory Signaling.
All olfactory neurons that express the same odorant receptor are thought to project to a single glomerulus on either side of the olfactory bulb (22, 23). The localization of PDE2 and GC-D in a subset of olfactory neurons that project to a unique group of glomeruli suggests that they are involved in a function distinct from that of other olfactory neurons. These glomeruli have been identified as the necklace glomeruli based on the presence of high AChE activity in nerve endings originating from the brain (24) and the presence of olfactory nerve terminals expressing PAX (20). The modified glomerular complex, one of the necklace glomeruli, has been associated with suckling behavior, a pheromone induced response, in rat pups (26, 27). Other studies, however, suggest that this glomerular complex is not wholly responsible for this behavior in that obliteration of nerve input to the modified glomerular complex does not inhibit suckling behavior (29, 30). It is possible, however, that the necklace functions as a whole and it might be necessary to obliterate the entire necklace before observing an effect.
These observations suggest the possibility that the function for this group of neurons may be in behavioral responses induced by hormones or pheromones, possibly related to reproduction, rather than a response to specific odorants. This concept is further supported by the presence of centrifugal innervation of the modified glomerular complex by luteinizing-hormone releasing hormone expressing nerve terminals (31, 32) and by the presence of PAX (20). Several studies have indicated that olfactory neurons can be responsible for some of the behavioral responses that have been generally attributed to the vomeronasal organ and the accessory olfactory bulb (33, 34).
At this time we know that these neurons do not contain detectable levels of ACIII and PDE1C2. Both of these proteins are thought to be important components of odorant signaling. We do not know, however, which other putative components of the odorant response, if any, are present in these neurons; for example, an odorant receptor or the cyclic nucleotide gated channel (see Fig. 9). It could be that the cAMP-mediated pathway present in these neurons is stimulated by a pheromone or some other hormone receptor that is modulated by cGMP via GC-D and PDE2. Since no ligand has yet been identified for GC-D, it is difficult to do more than speculate; however, it is possible that GC-D may itself be a pheromone or hormone receptor. PDE2 will hydrolyze cGMP as well as cAMP and its main purpose may be in negative feedback. Taken together the data suggest that these neurons perform a unique function specifically modulated by cGMP.
Compartmentalization of PDE1C2, ACIII, and PDE4A in Olfactory Neurons.
PDE1C2, ACIII, and PDE4A are expressed in what appear to be a large majority of olfactory neurons with the exception of the group of neurons containing PDE2 and GC-D. It is possible, however, that there are other subsets or individual olfactory neurons that do not contain any of these proteins. ACIII and PDE1C2 are thought to be responsible for initiation and rapid decline, respectively, of the spike of cAMP seen in most olfactory neurons upon presentation of an odorant. Both of these proteins are found in high concentrations in the same cilia in close proximity to the putative odorant receptors with little to no expression detected in cell bodies and axons by immunohistochemistry.
The PDE4A isoforms specifically hydrolyze cAMP with a high affinity. To date at least three known splice variants of PDE4A have been identified in rats (35, 36). In contrast to PDE1C2 and ACIII, PDE4A is found in the dendritic knobs, cell bodies, and at very high concentrations in the axons of olfactory neurons with no expression in olfactory cilia. Colocalization studies using antisera against PDE4A and ACIII or PDE1C2 show that these enzymes are found in the same neurons but in different subcellular compartments. No function has been determined for PDE4A in olfactory neurons. Its major role, however, might be associated with transmission of the odorant signal to the olfactory bulb because it is found at high concentrations in the axons of olfactory neurons and is absent from the subset of neurons expressing PDE2 and GC-D.
The distinct localization of PDE1C2, ACIII, and PDE4A in the same olfactory neurons but different compartments strongly suggests that there are uniquely regulated pools of cAMP within the same cell. At a minimum, one pool that is produced and hydrolyzed in the cilia by ACIII and PDE1C2, respectively, and one that is hydrolyzed by PDE4A in the dendrites, cell bodies, and axons. These data also suggest that there may be another isoform of adenylyl cyclase in the cell bodies and axons of these neurons since PDE4A hydrolyzes only cAMP. In conclusion, the data presented here strongly suggest that selective compartmentalization of different PDEs and cyclases within and between neurons are an important feature for the regulation of signal transduction in olfactory neurons and probably in other neurons as well.
Acknowledgments
We thank Setareh Seraji for technical assistance, and Greg Jensen and Sergio Martinez for supplying purified Sf9-cell expressed PDE2. Confocal microscopy and image analysis was performed in the Imaging Facility of the W. M. Keck Center for Advanced Studies of Neuronal Signaling (University of Washington). This work was supported by grants from the National Institutes of Health (DK21723) to J.A.B., and the Medical Research Council (United Kingdom) to M.D.H.
ABBREVIATIONS
- PDE
phosphodiesterase
- PDE2
cGMP-stimulated phosphodiesterase
- GC-D
olfactory specific guanylyl cyclase, PDE1C2, the 1C2 isozyme of calcium/calmodulin-dependent phosphodiesterase
- PDE4A
4A subfamily of cAMP-specific phosphodiesterase
- ACIII
adenylyl cyclase III
- PAX
placental antigen
- AChE
acetylcholinesterase
References
- 1.Brunet L J, Gold G H, Ngai J. Neuron. 1996;17:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 2.Buck L, Axel R. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 3.Pace U, Hanski E, Salomon Y, Lancet D. Nature (London) 1985;316:255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- 4.Bakalyar H A, Reed R R. Science. 1990;250:1403–1406. doi: 10.1126/science.2255909. [DOI] [PubMed] [Google Scholar]
- 5.Jones D T, Reed R R. Science. 1989;244:790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Gold G H. Nature (London) 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- 7.Dhallan R S, Yau K W, Schrader K A, Reed R R. Nature (London) 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- 8.Fülle H J, Vassar R, Foster D C, Yang R B, Axel R, Garbers D L. Proc Natl Acad Sci USA. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fülle H J, Garbers D L. Cell Biochem Funct. 1994;12:157–165. doi: 10.1002/cbf.290120303. [DOI] [PubMed] [Google Scholar]
- 10.Ressler K J, Sullivan S L, Buck L B. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- 11.Vassar R, Ngai J, Axel R. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- 12.Yu S, Avery L, Baude E, Garbers D L. Proc Natl Acad Sci USA. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beavo J A. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 14.Borisy F F, Ronnett G V, Cunningham A M, Juilfs D, Beavo J, Snyder S H. J Neurosci. 1992;12:915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan C, Zhao A Z, Bentley J K, Loughney K, Ferguson K, Beavo J A. Proc Natl Acad Sci USA. 1995;92:9677–9681. doi: 10.1073/pnas.92.21.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherry J A, Davis R L. J Neurobiol. 1995;28:102–113. doi: 10.1002/neu.480280109. [DOI] [PubMed] [Google Scholar]
- 17.Jensenius J C, Andersen I, Hau J, Crone M, Koch C. J Immunol Methods. 1981;46:63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- 18.Smith D E, Fisher P A. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakur Y, Wilson M, Pooley L, Lobban M, Griffiths S L, Campbell A M, Beattie J, Daly C, Houslay M D. Biochem J. 1995;306:801–809. doi: 10.1042/bj3060801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinoda K, Ohtsuki T, Nagano M, Okumura T. Brain Res. 1993;618:160–166. doi: 10.1016/0006-8993(93)90440-x. [DOI] [PubMed] [Google Scholar]
- 21.Sonnenburg W K, Mullaney P J, Beavo J A. J Biol Chem. 1991;266:17655–17661. [PubMed] [Google Scholar]
- 22.Vassar R, Chao S K, Sitcheran R, Nunez J M, Vosshall L B, Axel R. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 23.Ressler K J, Sullivan S L, Buck L B. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 24.Zheng L M, Ravel N, Jourdan F. Neuroscience. 1987;23:1083–1093. doi: 10.1016/0306-4522(87)90183-7. [DOI] [PubMed] [Google Scholar]
- 25.Shinoda K, Shiotani Y, Osawa Y. J Comp Neurol. 1989;284:362–373. doi: 10.1002/cne.902840304. [DOI] [PubMed] [Google Scholar]
- 26.Teicher M H, Stewart W B, Kauer J S, Shepherd G M. Brain Res. 1980;194:530–535. doi: 10.1016/0006-8993(80)91237-8. [DOI] [PubMed] [Google Scholar]
- 27.Greer C A, Stewart W B, Teicher M H, Shepherd G M. J Neurosci. 1982;2:1744–1759. doi: 10.1523/JNEUROSCI.02-12-01744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trong H L, Beier N, Sonnenburg W K, Stroop S D, Walsh K A, Beavo J A, Charbonneau H. Biochemistry. 1990;29:10280–10288. doi: 10.1021/bi00496a018. [DOI] [PubMed] [Google Scholar]
- 29.Hudson R, Distel H. Brain Res. 1987;421:85–94. doi: 10.1016/0006-8993(87)91278-9. [DOI] [PubMed] [Google Scholar]
- 30.Risser J M, Slotnick B M. Brain Res Bull. 1987;19:275–281. doi: 10.1016/0361-9230(87)90093-1. [DOI] [PubMed] [Google Scholar]
- 31.Rosser A E, Hokfelt T, Goldstein M. J Comp Neurol. 1986;250:352–363. doi: 10.1002/cne.902500308. [DOI] [PubMed] [Google Scholar]
- 32.Zheng L M, Caldani M, Jourdan F. Neuroscience. 1988;24:567–578. doi: 10.1016/0306-4522(88)90350-8. [DOI] [PubMed] [Google Scholar]
- 33.Romeyer A, Poindron P, Orgeur P. Physiol Behav. 1994;56:693–700. doi: 10.1016/0031-9384(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 34.L’evy F, Locatelli A, Piketty V, Tillet Y, Poindron P. Physiol Behav. 1995;57:97–104. doi: 10.1016/0031-9384(94)00200-o. [DOI] [PubMed] [Google Scholar]
- 35.Bolger G B. Cell Signalling. 1994;6:851–859. doi: 10.1016/0898-6568(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 36.Conti M, Nemoz G, Sette C, Vicini E. Endocr Rev. 1995;16:370–389. doi: 10.1210/edrv-16-3-370. [DOI] [PubMed] [Google Scholar]