Abstract
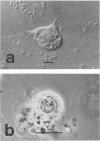
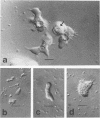
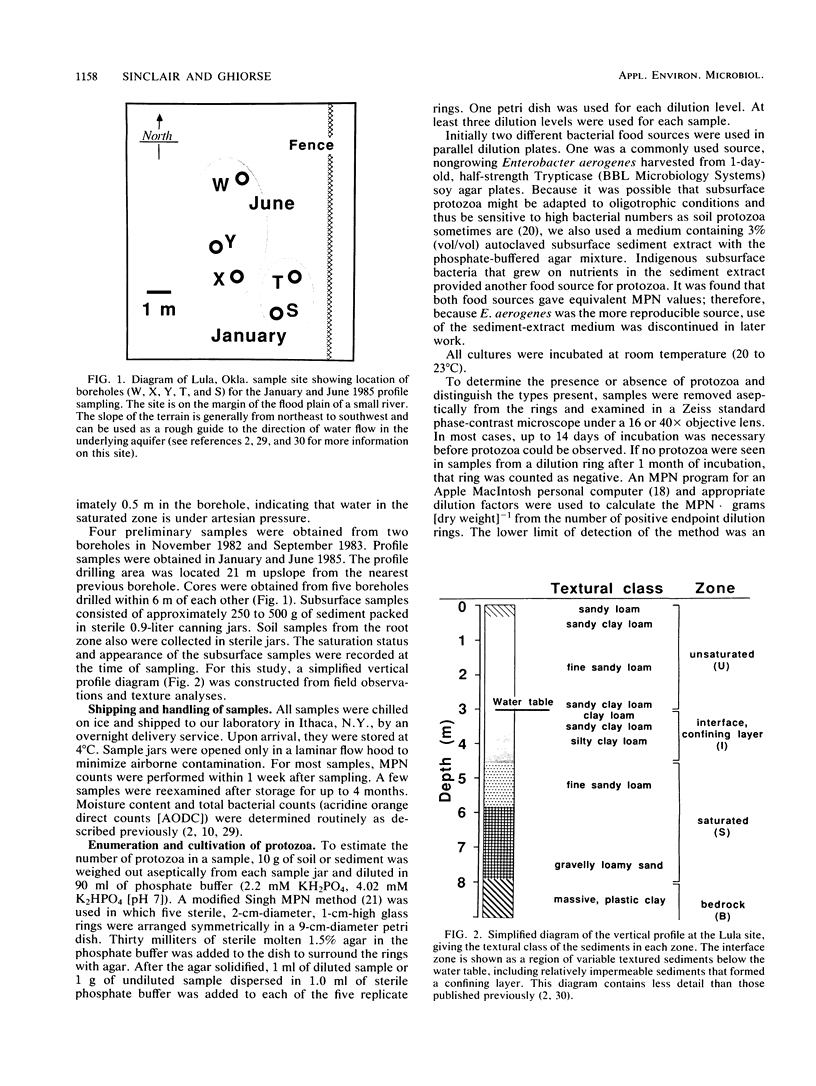
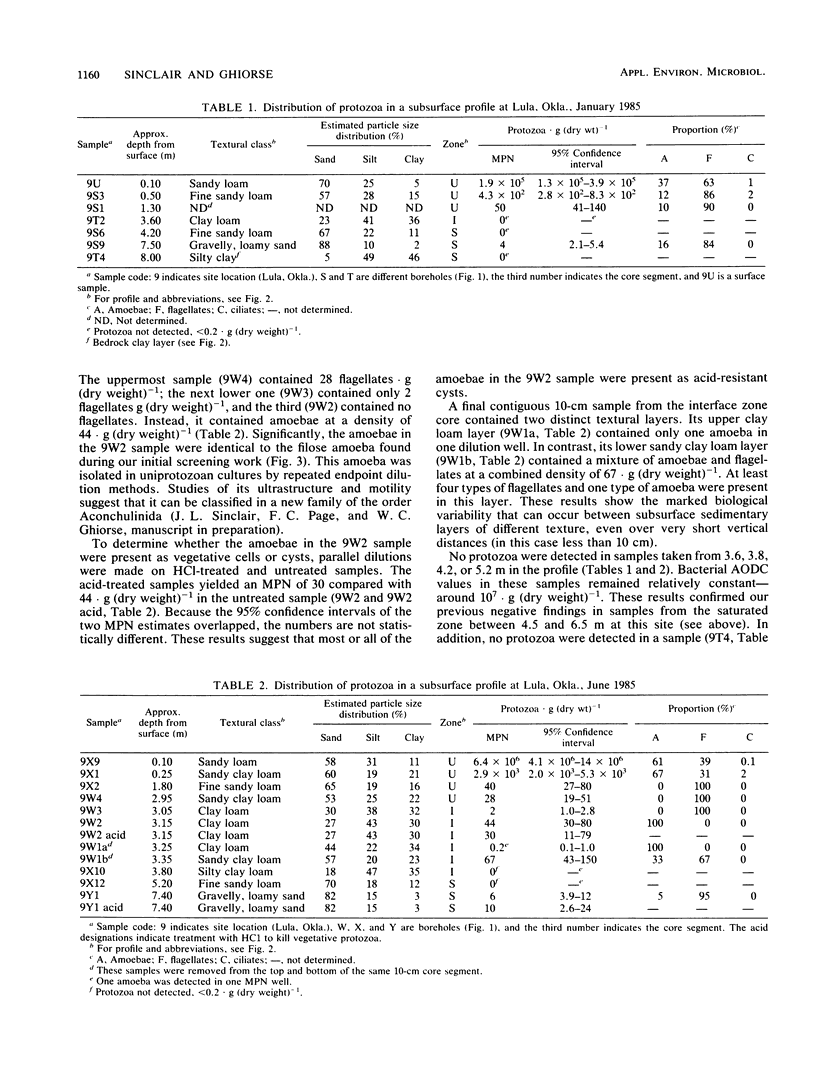
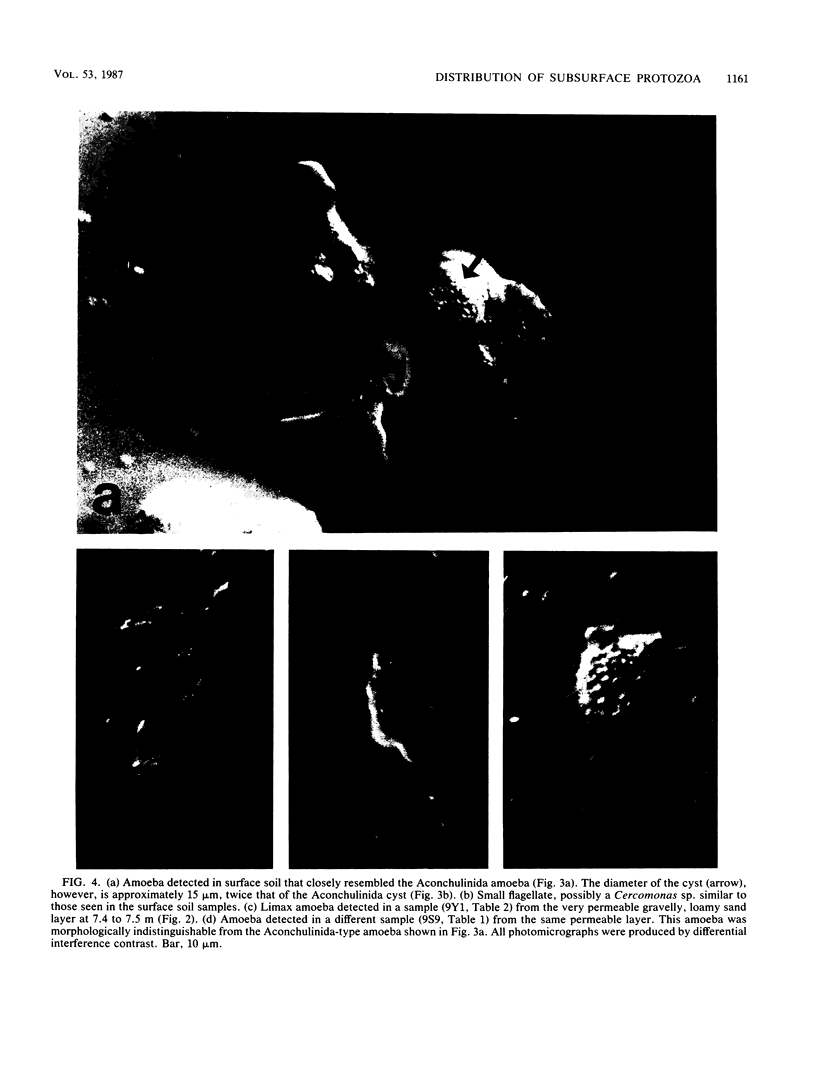
Sediment core samples were obtained at a groundwater study site in Oklahoma in January and June 1985. Most-probable-number estimates showed that protozoan numbers declined steeply with depth in subsoil. Flagellates and amoebae dominated the protozoan population, which declined to a most probable number of 28 · g (dry weight)−1 in a clay loam layer at the bottom of the unsaturated zone. Samples from a texturally variable interface zone between 3 and 4 m down also were variable in their content of protozoa. Four contiguous clay loam samples in a single core from this zone contained variable numbers of amoebae ranging from 0.2 to 44 · g (dry weight)−1. However, a sandy clay loam layer at the bottom of the core contained a mixture of flagellates and amoebae with a combined population density of 67 · g (dry weight)−1. A slow-growing filose amoeba was isolated from interface zone samples and was tentatively classified in a new family in the order Aconchulinida. Protozoa were not detected in the saturated zone except in a very permeable gravelly, loamy sand layer at a depth of approximately 7.5 m. Low numbers (4 to 6 · g [dry weight]−1) of surface-type flagellates and amoebae, as well as the filose amoeba seen in the interface zone, were observed in this layer. Acid-treated and untreated samples contained equivalent numbers of protozoa, showing that the majority of protozoa in the layer at 7.5 m and the interface zone samples were encysted. Increased numbers of bacteria also were found in the layer at 7.5 m, indicating that it was biologically more active than other saturated-zone layers. Cyanobacteria grew in illuminated samples from this layer, suggesting that it may be connected hydrologically to a nearby river.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill D. L., Ghiorse W. C. Characterization of subsurface bacteria associated with two shallow aquifers in oklahoma. Appl Environ Microbiol. 1985 Sep;50(3):580–588. doi: 10.1128/aem.50.3.580-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone T. L., Balkwill D. L. Improved flotation technique for microscopy of in situ soil and sediment microorganisms. Appl Environ Microbiol. 1986 Mar;51(3):462–468. doi: 10.1128/aem.51.3.462-468.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofoid C. A. ON THE RELATIVE NUMIBERS OF RHIZOPODS AND FLAGELLATES IN THE FAUNA OF SOILS. Science. 1915 Dec 31;42(1096):937–940. doi: 10.1126/science.42.1096.937-a. [DOI] [PubMed] [Google Scholar]
- Russek E., Colwell R. R. Computation of most probable numbers. Appl Environ Microbiol. 1983 May;45(5):1646–1650. doi: 10.1128/aem.45.5.1646-1650.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]