Abstract
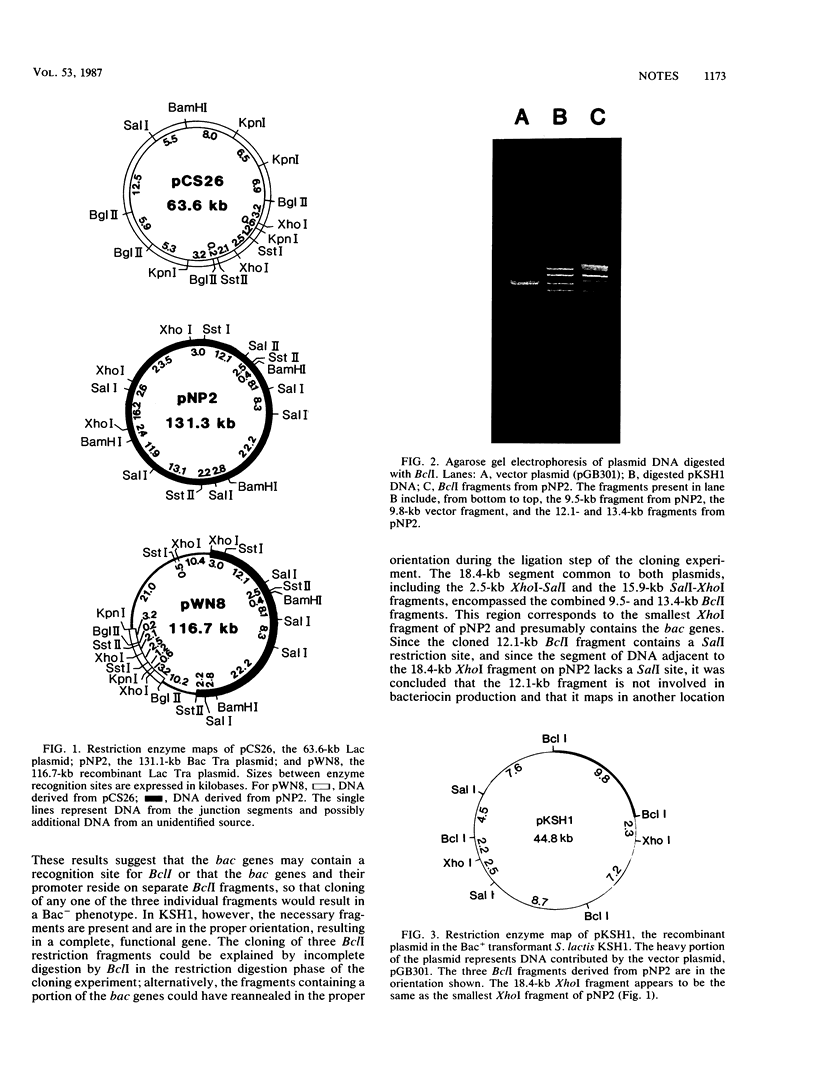
The 131.1-kilobase (kb) bacteriocin production (Bac) plasmid pNP2 and the 63.6-kb lactose metabolism (Lac) plasmid pCS26, from Streptococcus lactis subsp. diacetylactis WM4, as well as pWN8, a 116.7-kb recombinant plasmid from a Lac+ transconjugant, were analyzed with restriction enzymes to determine the origin of pWN8. Plasmid pWN8 conferred a Lac+ Bac- phenotype, contained DNA derived from pCS26 and pNP2, and, like pNP2, exhibited self-transmissibility (Tra+). In cloning attempts, Bac+ transformant S. lactis KSH1 was isolated. The recombinant plasmid, pKSH1, contained three BclI fragments from pNP2. Bac- transformants which individually contained each of the three fragments were also identified. Comparison of restriction maps of pKSH1 and pNP2 revealed an 18.4-kb region common to both plasmids, involving two of the three BclI fragments. S. lactis KSH1 also exhibited greater inhibitory activity against the indicator strain S. diacetylactis 18-16 than did a strain containing the 131.1-kb Bac plasmid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Genetic and physical characterization of recombinant plasmids associated with cell aggregation and high-frequency conjugal transfer in Streptococcus lactis ML3. J Bacteriol. 1984 Jun;158(3):954–962. doi: 10.1128/jb.158.3.954-962.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Gilmore M. S., Ferretti J. J. Plasmid pGB301, a new multiple resistance streptococcal cloning vehicle and its use in cloning of a gentamicin/kanamycin resistance determinant. Mol Gen Genet. 1981;182(3):414–421. doi: 10.1007/BF00293929. [DOI] [PubMed] [Google Scholar]
- Davey G. P. Plasmid associated with diplococcin production in Streptococcus. Appl Environ Microbiol. 1984 Oct;48(4):895–896. doi: 10.1128/aem.48.4.895-896.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J., Davies F. L. High-frequency conjugation associated with Streptococcus lactis donor cell aggregation. J Bacteriol. 1980 Sep;143(3):1260–1264. doi: 10.1128/jb.143.3.1260-1264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo J. K., McKay L. L. Transformation of Streptococcus lactis Protoplasts by Plasmid DNA. Appl Environ Microbiol. 1982 May;43(5):1213–1215. doi: 10.1128/aem.43.5.1213-1215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kékessy D. A., Piguet J. D. New method for detecting bacteriocin production. Appl Microbiol. 1970 Aug;20(2):282–283. doi: 10.1128/am.20.2.282-283.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh G. L., Swartz M. N. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob Agents Chemother. 1977 Sep;12(3):423–426. doi: 10.1128/aac.12.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Walsh P. M. Conjugal transfer of genetic information in group N streptococci. Appl Environ Microbiol. 1980 Jul;40(1):84–89. doi: 10.1128/aem.40.1.84-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Cords B. R., Baldwin K. A. Transduction of lactose metabolism in Streptococcus lactis C2. J Bacteriol. 1973 Sep;115(3):810–815. doi: 10.1128/jb.115.3.810-815.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve H., Geis A., Teuber M. Conjugal transfer and characterization of bacteriocin plasmids in group N (lactic acid) streptococci. J Bacteriol. 1984 Mar;157(3):833–838. doi: 10.1128/jb.157.3.833-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherwitz K. M., Baldwin K. A., McKay L. L. Plasmid linkage of a bacteriocin-like substance in Streptococcus lactis subsp. diacetylactis strain WM4: transferability to Streptococcus lactis. Appl Environ Microbiol. 1983 May;45(5):1506–1512. doi: 10.1128/aem.45.5.1506-1512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J. L., McKay L. L. Partial characterization of the genetic basis for sucrose metabolism and nisin production in Streptococcus lactis. Appl Environ Microbiol. 1986 Jan;51(1):57–64. doi: 10.1128/aem.51.1.57-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. M., McKay L. L. Recombinant plasmid associated cell aggregation and high-frequency conjugation of Streptococcus lactis ML3. J Bacteriol. 1981 Jun;146(3):937–944. doi: 10.1128/jb.146.3.937-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]