Abstract
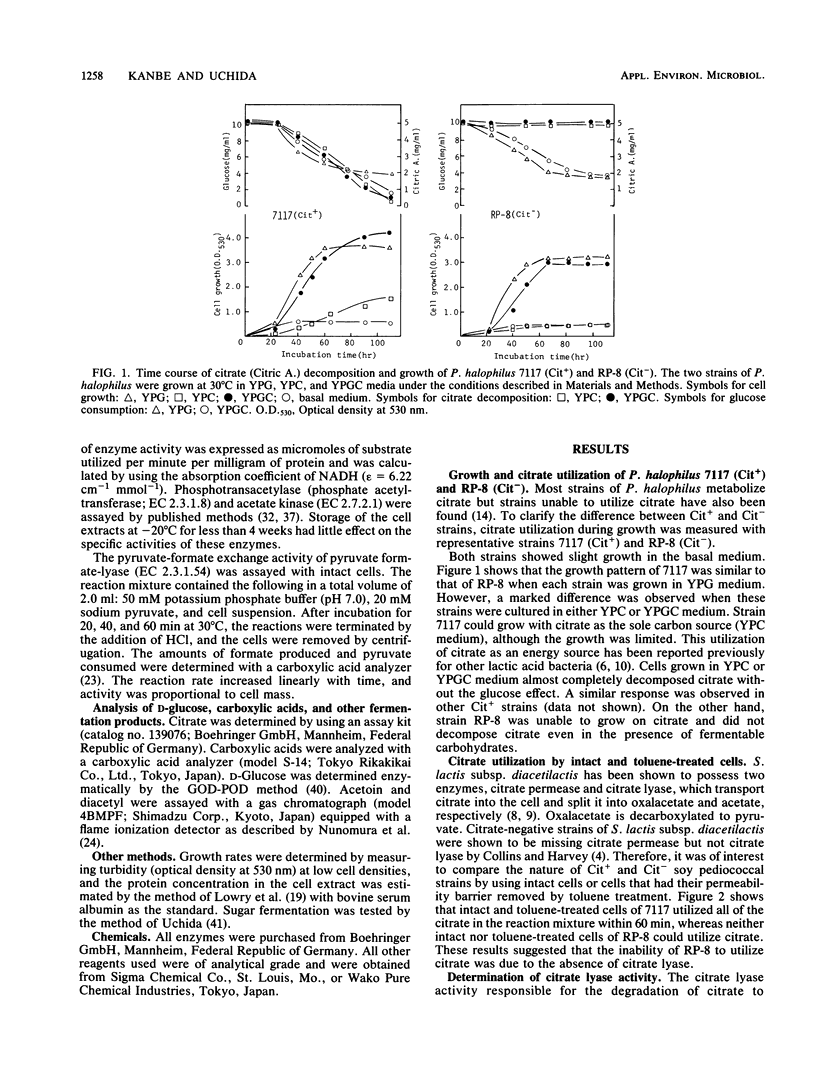
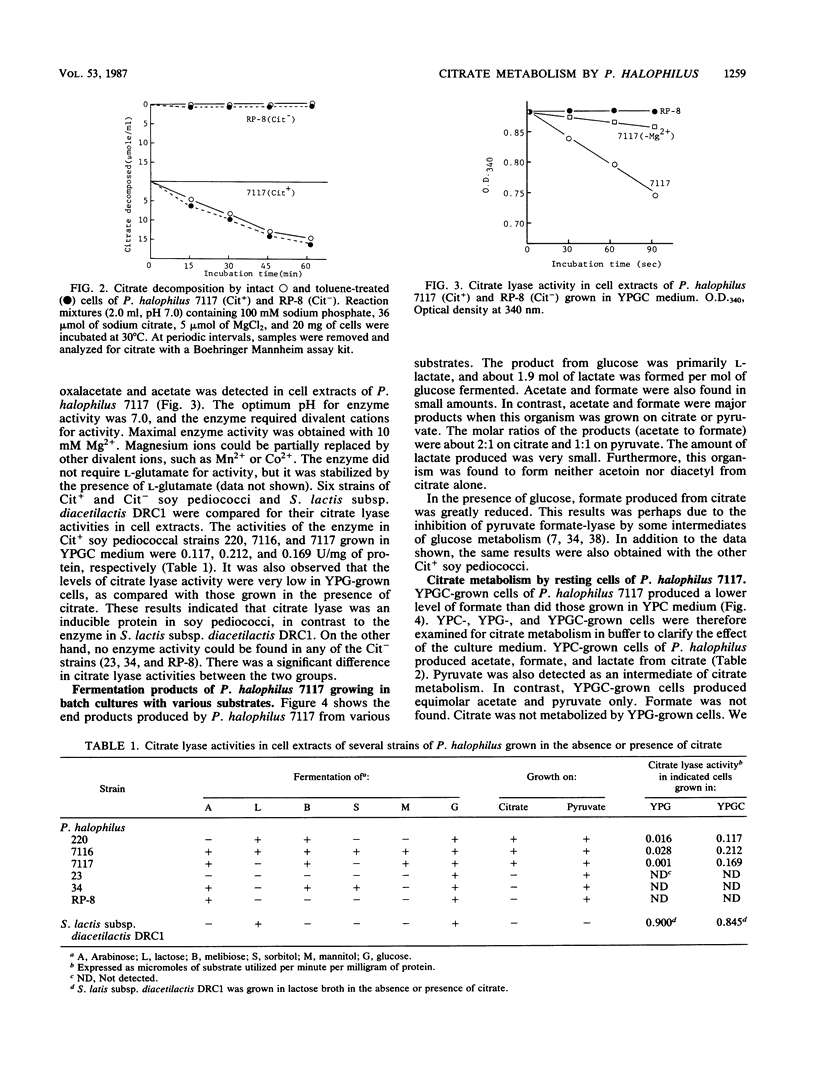
Several strains of non-citrate-metabolizing Pediococcus halophilus have previously been isolated from soy sauce mash or moromi. The factors controlling the metabolism of citrate in soy pediococci were studied. All the soy pediococcal strains tested which failed to decompose citrate did not possess citrate lyase [citrate (pro-3S)-lyase; EC 4.1.3.6] activity. In P. halophilus, citrate lyase was an inducible enzyme, and the optimum pH for activity was 7.0. The metabolism of citrate in P. halophilus was different from that observed in lactic streptococci. The main products from citrate were acetate and formate, and this bacterium produced no acetoin or diacetyl. Formate production from citrate was greatly reduced in the presence of glucose. P. halophilus 7117 (Cit+) was proved to contain citrate lyase, pyruvate formate-lyase (EC 2.3.1.54) phosphotransacetylase (phosphate acetyltransferase; EC 2.3.1.8), and acetate kinase (EC 2.7.2.1), i.e., all the enzymes necessary to convert citrate to acetate and formate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Involvement of oxygen-sensitive pyruvate formate-lyase in mixed-acid fermentation by Streptococcus mutans under strictly anaerobic conditions. J Bacteriol. 1982 Oct;152(1):175–182. doi: 10.1128/jb.152.1.175-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinan D. F., Robin S., Cogan T. M. Citric acid metabolism in hetero- and homofermentative lactic acid bacteria. Appl Environ Microbiol. 1976 Apr;31(4):481–486. doi: 10.1128/aem.31.4.481-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce A. M., Crow V. L., Thomas T. D. Regulation of product formation during glucose or lactose limitation in nongrowing cells of Streptococcus lactis. Appl Environ Microbiol. 1984 Aug;48(2):332–337. doi: 10.1128/aem.48.2.332-337.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. Citrate transport system of Streptococcus diacetilactis. J Bacteriol. 1962 May;83:1005–1009. doi: 10.1128/jb.83.5.1005-1009.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. ROLES OF CITRATE AND ACETOIN IN THE METABOLISM OF STREPTOCOCCUS DIACETILACTIS. J Bacteriol. 1963 Dec;86:1301–1307. doi: 10.1128/jb.86.6.1301-1307.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. Role of citritase in acetoin formation by Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1961 Dec;82:954–959. doi: 10.1128/jb.82.6.954-959.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M. W., Hillier A. J., Jago G. R. Metabolism of pyruvate and citrate in lactobacilli. Aust J Biol Sci. 1983;36(5-6):487–496. doi: 10.1071/bi9830487. [DOI] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Characterization of Plasmid Deoxyribonucleic Acid in Streptococcus lactis subsp. diacetylactis: Evidence for Plasmid-Linked Citrate Utilization. Appl Environ Microbiol. 1979 Feb;37(2):316–323. doi: 10.1128/aem.37.2.316-323.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmel A., Behrens G., Gottschalk G. Citrate lyase from Streptococcus diacetilactis. Association with its acetylating enzyme. Arch Microbiol. 1975;102(2):111–116. doi: 10.1007/BF00428354. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindmark D. G., Paolella P., Wood N. P. The pyruvate formate-lyase system of Streptococcus faecalis. I. Purification and properties of the formate-pyruvate exchange enzyme. J Biol Chem. 1969 Jul 10;244(13):3605–3612. [PubMed] [Google Scholar]
- McKay L. L. Functional properties of plasmids in lactic streptococci. Antonie Van Leeuwenhoek. 1983 Sep;49(3):259–274. doi: 10.1007/BF00399502. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Ozawa Y., Tamura Z. A highly efficient carboxylic acid analyzer and its application. J Chromatogr. 1976 Jul 21;123(1):129–138. doi: 10.1016/s0021-9673(00)81109-7. [DOI] [PubMed] [Google Scholar]
- O'Brien Induction by sodium of the citrate fermentation enzymes in Klebsiella aerogenes. FEBS Lett. 1975 Apr 15;53(1):61–63. doi: 10.1016/0014-5793(75)80682-x. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W. Induction of citrate lyase in Enterobacter cloacae grown under aerated conditions and its effect on citrate metabolism. J Bacteriol. 1975 Dec;124(3):1084–1088. doi: 10.1128/jb.124.3.1084-1088.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radler F., Bröhl K. The metabolism of several carboxylic acids by lactic acid bacteria. Z Lebensm Unters Forsch. 1984 Sep;179(3):228–231. doi: 10.1007/BF01041899. [DOI] [PubMed] [Google Scholar]
- Rhee S. K., Pack M. Y. Effect of environmental pH on fermentation balance of Lactobacillus bulgaricus. J Bacteriol. 1980 Oct;144(1):217–221. doi: 10.1128/jb.144.1.217-221.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensäter G., Takahashi-Abbe S., Abbe K., Birkhed D., Yamada T., Edwardsson S. Anaerobic and aerobic metabolism of sorbitol in Streptococcus sanguis and Streptococcus mitior. J Dent Res. 1985 Nov;64(11):1286–1289. doi: 10.1177/00220345850640110601. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Abbe K., Yamada T. Purification of pyruvate formate-lyase from Streptococcus mutans and its regulatory properties. J Bacteriol. 1982 Mar;149(3):1034–1040. doi: 10.1128/jb.149.3.1034-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Turner K. W., Crow V. L. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J Bacteriol. 1980 Nov;144(2):672–682. doi: 10.1128/jb.144.2.672-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Takahashi-Abbe S., Abbe K. Effects of oxygen on pyruvate formate-lyase in situ and sugar metabolism of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1985 Jan;47(1):129–134. doi: 10.1128/iai.47.1.129-134.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


