Abstract
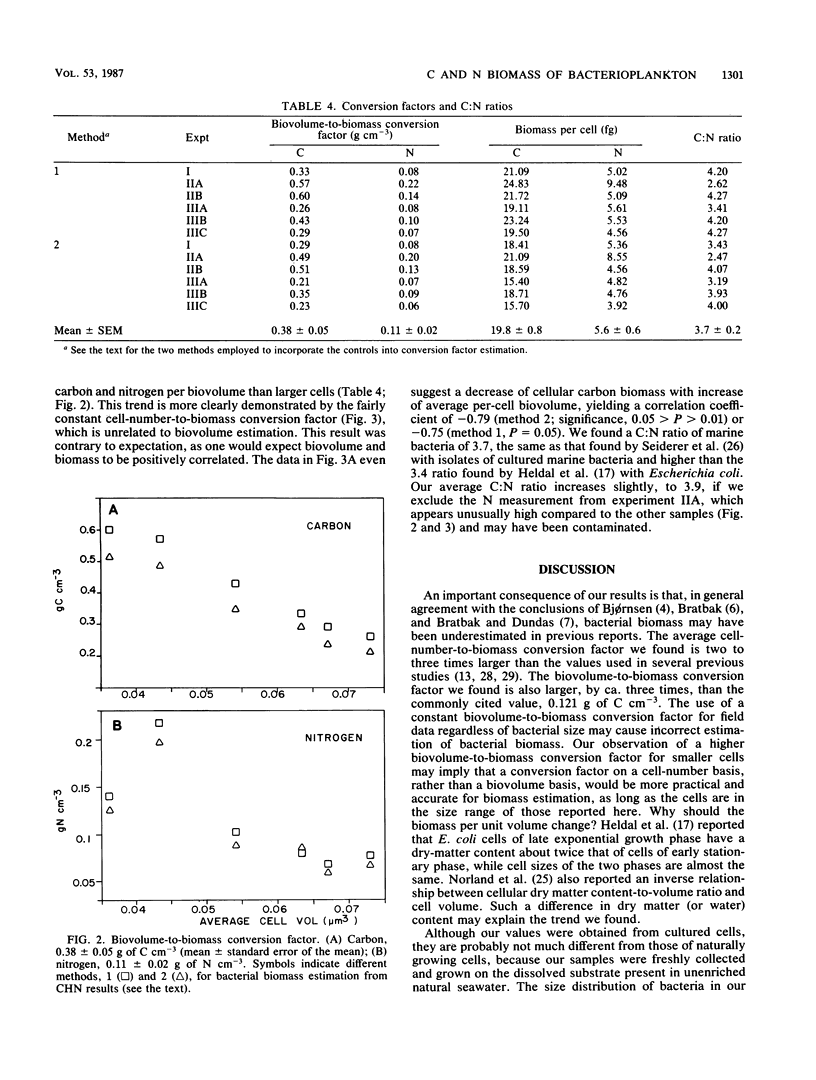
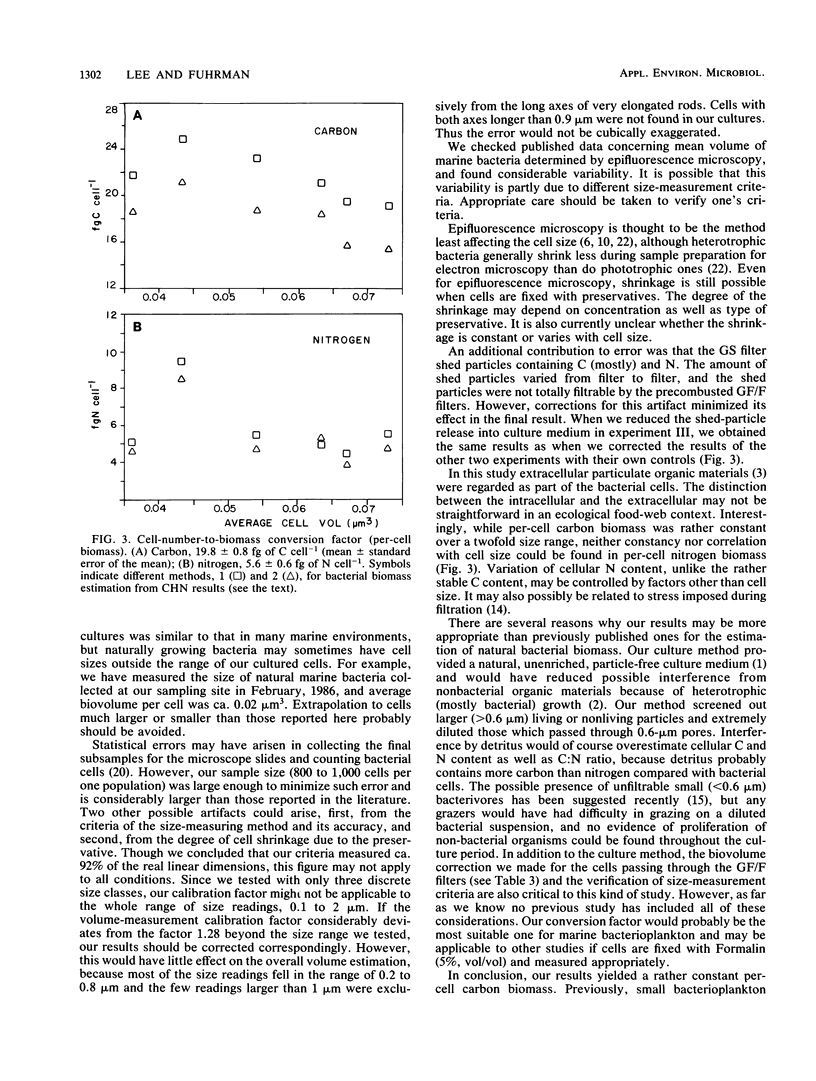
Microscopic estimation of bacterial biomass requires determination of both biovolume and biovolume-to-biomass conversion. Both steps have uncertainty when applied to the very small bacteria typically found in natural seawater. In the present study, natural bacterioplankton assemblages were freshly collected, passed through 0.6-μm-pore-size Nuclepore filters to remove larger particulate materials, and diluted for growth in 0.22-μm-pore-size Millipore filter-sterilized unenriched seawater. This provided cells comparable in size and morphology to those in natural seawater, but the cultures were free of the interfering particulate detritus naturally present. Cells were collected on glass-fiber GF/F filters, and biovolumes were corrected for cells passing these filters; C and N were measured with a CHN analyzer. Our criteria for size measurement by epifluorescence photomicrography were confirmed with fluorescent microspheres of known diameters. Surprisingly, in six cultures with average per-cell biovolumes ranging from 0.036 to 0.073 μm3, the average per-cell carbon biomass was relatively constant at 20 ± 0.08 fg of C (mean ± standard error of the mean). The biovolume-to-biomass conversion factor averaged 0.38 ± 0.05 g of C cm−3, which is about three times higher than the value previously estimated from Escherichia coli, and decreased with increasing cell volume. The C:N ratio was 3.7 ± 0.2. We conclude that natural marine bacterial biomass and production may be higher than was previously thought and that variations in bacterial size may not reflect variations in biomass per cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakken L. R., Olsen R. A. Buoyant densities and dry-matter contents of microorganisms: conversion of a measured biovolume into biomass. Appl Environ Microbiol. 1983 Apr;45(4):1188–1195. doi: 10.1128/aem.45.4.1188-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnsen P. K. Automatic determination of bacterioplankton biomass by image analysis. Appl Environ Microbiol. 1986 Jun;51(6):1199–1204. doi: 10.1128/aem.51.6.1199-1204.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden W. B. Comparison of two direct-count techniques for enumerating aquatic bacteria. Appl Environ Microbiol. 1977 May;33(5):1229–1232. doi: 10.1128/aem.33.5.1229-1232.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G. Bacterial biovolume and biomass estimations. Appl Environ Microbiol. 1985 Jun;49(6):1488–1493. doi: 10.1128/aem.49.6.1488-1493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G., Dundas I. Bacterial dry matter content and biomass estimations. Appl Environ Microbiol. 1984 Oct;48(4):755–757. doi: 10.1128/aem.48.4.755-757.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., McManus G. B. Do bacteria-sized marine eukaryotes consume significant bacterial production? Science. 1984 Jun 15;224(4654):1257–1260. doi: 10.1126/science.224.4654.1257. [DOI] [PubMed] [Google Scholar]
- Hagström A., Larsson U., Hörstedt P., Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979 May;37(5):805–812. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldal M., Norland S., Tumyr O. X-ray microanalytic method for measurement of dry matter and elemental content of individual bacteria. Appl Environ Microbiol. 1985 Nov;50(5):1251–1257. doi: 10.1128/aem.50.5.1251-1257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Sigda J., Kapuscinski R., Mitchell R. Statistical analysis of the direct count method for enumerating bacteria. Appl Environ Microbiol. 1982 Aug;44(2):376–382. doi: 10.1128/aem.44.2.376-382.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos E., Esteve I., Guerrero R. Comparison between direct methods for determination of microbial cell volume: electron microscopy and electronic particle sizing. Appl Environ Microbiol. 1983 May;45(5):1651–1658. doi: 10.1128/aem.45.5.1651-1658.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T. Carbon and nitrogen content of natural planktonic bacteria. Appl Environ Microbiol. 1986 Jul;52(1):28–32. doi: 10.1128/aem.52.1.28-32.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell S. Y., Christian R. R. Frequency of dividing cells as an estimator of bacterial productivity. Appl Environ Microbiol. 1981 Jul;42(1):23–31. doi: 10.1128/aem.42.1.23-31.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]