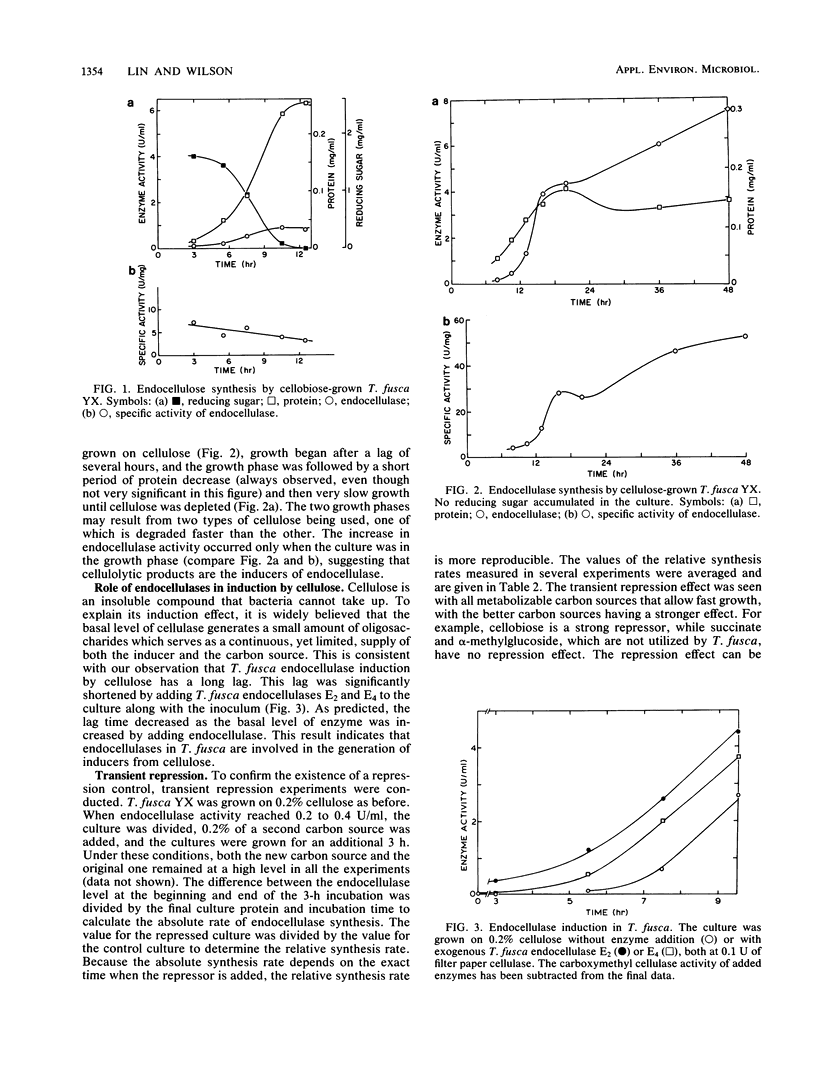
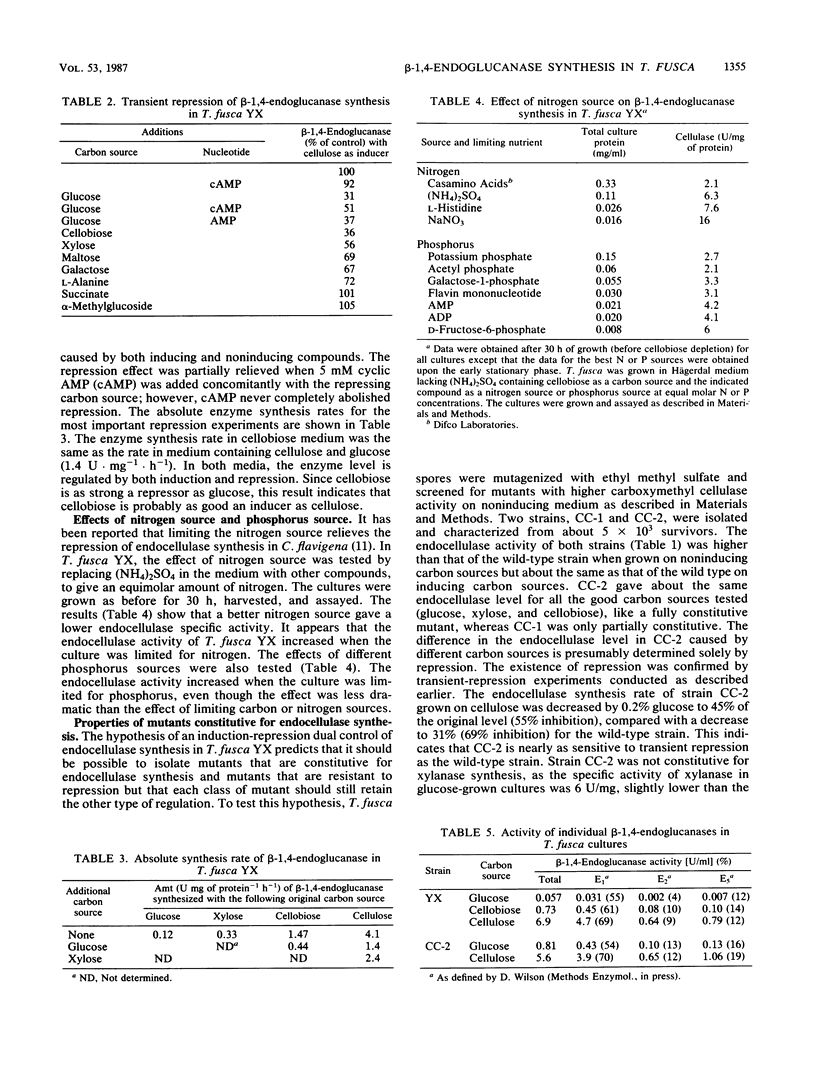
Abstract
In Thermomonospora fusca YX, endocellulase synthesis varies over a 100-fold range depending on the carbon source used. This study shows that the variation is caused by two regulatory mechanisms: an induction mechanism that increases the rate of endocellulase synthesis about 20-fold and a growth rate-dependent repression mechanism that changes the rate of synthesis over a 6-fold range in both induced and noninduced cells. In T. fusca, endocellulase synthesis can be induced by cellulose, cellobiose, or cellodextrin. Cellulase is involved in inducer generation from cellulose. Growth rate-dependent repression can be reversed by limiting cultures for carbon, nitrogen, or, to a lesser extent, phosphorus. Further evidence for two separate regulatory mechanisms is provided by the isolation of mutants (CC-1 and CC-2) whose endocellulases are synthesized constitutively but are still sensitive to growth rate-dependent repression. These conclusions about total endocellulase synthesis were extended to the individual endocellulases by showing that three T. fusca endocellulases are coordinately regulated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Breuil C., Kushner D. J. Cellulase induction and the use of cellulose as a preferred growth substrate by Cellvibrio gilvus. Can J Microbiol. 1976 Dec;22(12):1776–1781. doi: 10.1139/m76-264. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E. Cellulases of fungi. Basic Life Sci. 1981;18:19–32. doi: 10.1007/978-1-4684-3980-9_3. [DOI] [PubMed] [Google Scholar]
- Fennington G., Neubauer D., Stutzenberger F. Cellulase biosynthesis in a catabolite repression-resistant mutant of Thermomonospora curvata. Appl Environ Microbiol. 1984 Jan;47(1):201–204. doi: 10.1128/aem.47.1.201-204.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägerdal B. G., Ferchak J. D., Pye E. K. Cellulolytic Enzyme System of Thermoactinomyces sp. Grown on Microcrystalline Cellulose. Appl Environ Microbiol. 1978 Oct;36(4):606–612. doi: 10.1128/aem.36.4.606-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Stewart B. J., Leatherwood J. M. Derepressed synthesis of cellulase by Cellulomonas. J Bacteriol. 1976 Nov;128(2):609–615. doi: 10.1128/jb.128.2.609-615.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppok W., Rapp P., Wagner F. Formation, Location, and Regulation of Endo-1,4-beta-Glucanases and beta-Glucosidases from Cellulomonas uda. Appl Environ Microbiol. 1982 Jul;44(1):44–53. doi: 10.1128/aem.44.1.44-53.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. E., Neubauer D. G., Stutzenberger F. J. Cyclic AMP levels during induction and repression of cellulase biosynthesis in Thermomonospora curvata. J Bacteriol. 1984 Dec;160(3):1047–1054. doi: 10.1128/jb.160.3.1047-1054.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


