Abstract
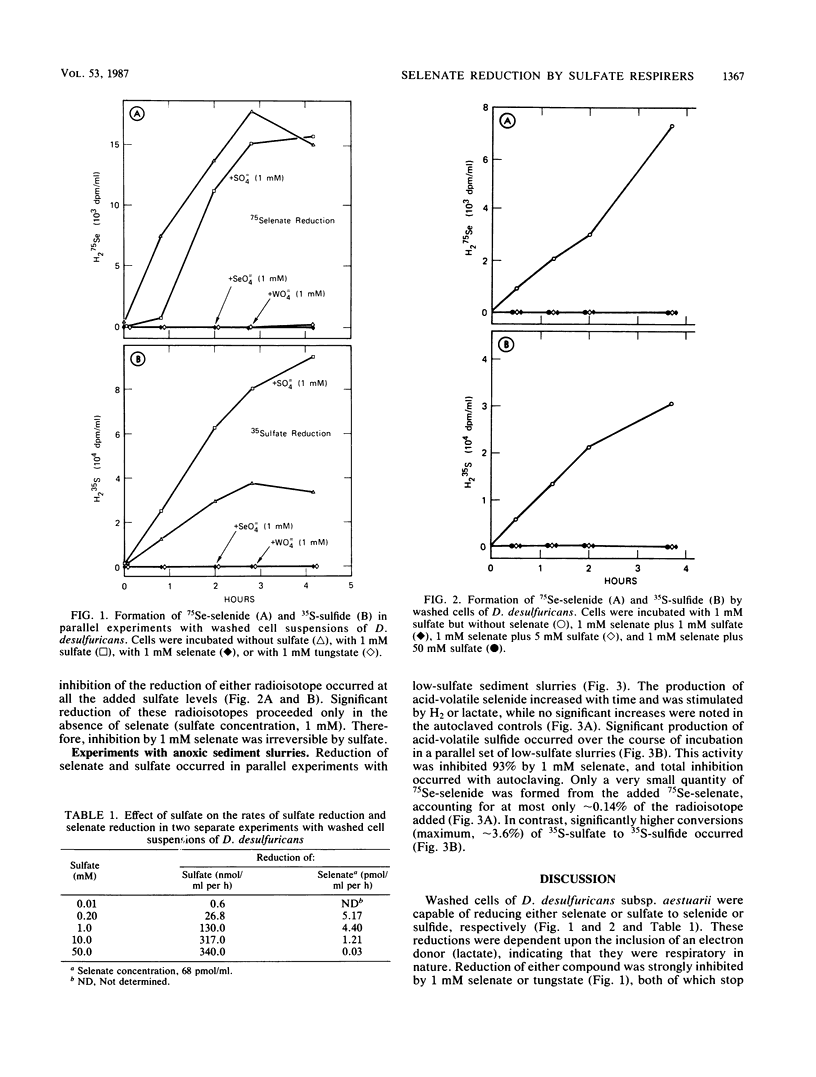
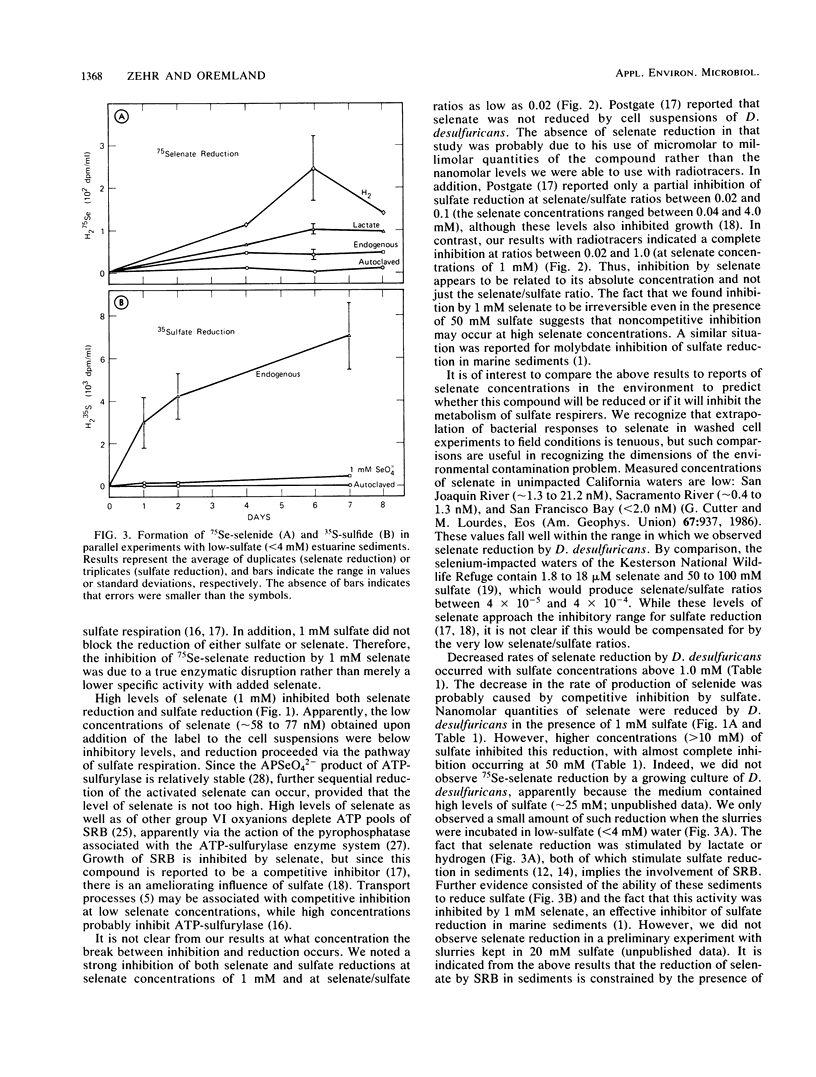
Washed cell suspensions of Desulfovibrio desulfuricans subsp. aestuarii were capable of reducing nanomolar levels of selenate to selenide as well as sulfate to sulfide. Reduction of these species was inhibited by 1 mM selenate or tungstate. The addition of 1 mM sulfate decreased the reduction of selenate and enhanced the reduction of sulfate. Increasing concentrations of sulfate inhibited rates of selenate reduction but enhanced sulfate reduction rates. Cell suspensions kept in 1 mM selenate were incapable of reducing either selenate or sulfate when the selenate/sulfate ratio was ≥0.02, indicating that irreversible inhibition occurs at high selenate concentrations. Anoxic estuarine sediments having an active flora of sulfate-respiring bacteria were capable of a small amount of selenate reduction when ambient sulfate concentrations were low (<4 mM). These results indicate that sulfate is an inhibitor of the reduction of trace quantities of selenate. Therefore, direct reduction of traces of selenate to selenide by sulfate-respiring bacteria in natural environments is constrained by the ambient concentration of sulfate ions. The significance of this observation with regard to the role sediments play in sequestering selenium is discussed.
Full text
PDF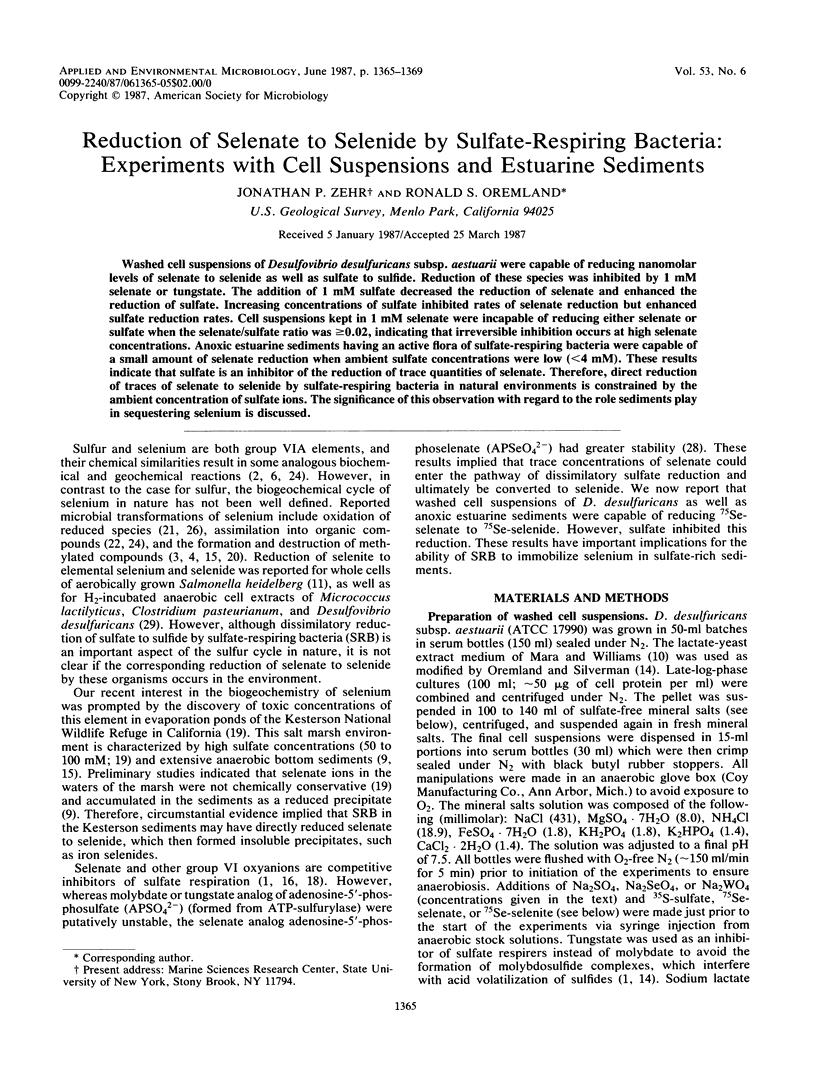


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doran J. W., Alexander M. Microbial transformations of selenium. Appl Environ Microbiol. 1977 Jan;33(1):31–37. doi: 10.1128/aem.33.1.31-37.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUSAKA C. Sulphate transport and metabolism by Desulphovibrio desulphuricans. Nature. 1961 Nov 4;192:427–429. doi: 10.1038/192427a0. [DOI] [PubMed] [Google Scholar]
- Francis A. J., Duxbury J. M., Alexander M. Evolution of dimethylselenide from soils. Appl Microbiol. 1974 Aug;28(2):248–250. doi: 10.1128/am.28.2.248-250.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mara D. D., Williams D. J. The evaluation of media used to enumerate sulphate reducing bacteria. J Appl Bacteriol. 1970 Sep;33(3):543–552. doi: 10.1111/j.1365-2672.1970.tb02232.x. [DOI] [PubMed] [Google Scholar]
- McCready R. G., Campbell J. N., Payne J. I. Selenite reduction by Salmonella heidelberg. Can J Microbiol. 1966 Aug;12(4):703–714. doi: 10.1139/m66-097. [DOI] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Zehr J. P. Formation of methane and carbon dioxide from dimethylselenide in anoxic sediments and by a methanogenic bacterium. Appl Environ Microbiol. 1986 Nov;52(5):1031–1036. doi: 10.1128/aem.52.5.1031-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R. Competitive and noncompetitive inhibitors of bacterial sulphate reduction. J Gen Microbiol. 1952 Feb;6(1-2):128–142. doi: 10.1099/00221287-6-1-2-128. [DOI] [PubMed] [Google Scholar]
- Peck H. D. THE ATP-DEPENDENT REDUCTION OF SULFATE WITH HYDROGEN IN EXTRACTS OF DESULFOVIBRIO DESULFURICANS. Proc Natl Acad Sci U S A. 1959 May;45(5):701–708. doi: 10.1073/pnas.45.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reamer D. C., Zoller W. H. Selenium biomethylation products from soil and sewage sludge. Science. 1980 May 2;208(4443):500–502. doi: 10.1126/science.208.4443.500. [DOI] [PubMed] [Google Scholar]
- Sarathchandra S. U., Watkinson J. H. Oxidation of elemental selenium to selenite by Bacillus megaterium. Science. 1981 Feb 6;211(4482):600–601. doi: 10.1126/science.6779378. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Reduction of sulfur compounds in the sediments of a eutrophic lake basin. Appl Environ Microbiol. 1981 May;41(5):1230–1237. doi: 10.1128/aem.41.5.1230-1237.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Science. 1974 Mar 8;183(4128):915–922. doi: 10.1126/science.183.4128.915. [DOI] [PubMed] [Google Scholar]
- Torma A. E., Habashi F. Oxidation of copper (II) selenide by Thiobacillus ferrooxidans. Can J Microbiol. 1972 Nov;18(11):1780–1781. doi: 10.1139/m72-278. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- WOOLFOLK C. A., WHITELEY H. R. Reduction of inorganic compounds with molecular hydrogen by Micrococcus lactilyticus. I. Stoichiometry with compounds of arsenic, selenium, tellurium, transition and other elements. J Bacteriol. 1962 Oct;84:647–658. doi: 10.1128/jb.84.4.647-658.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware D. A., Postgate J. R. Physiological and chemical properties of a reductant-activated inorganic pyrophosphatase from Desulfovibrio desulfuricans. J Gen Microbiol. 1971 Aug;67(2):145–160. doi: 10.1099/00221287-67-2-145. [DOI] [PubMed] [Google Scholar]



