Abstract
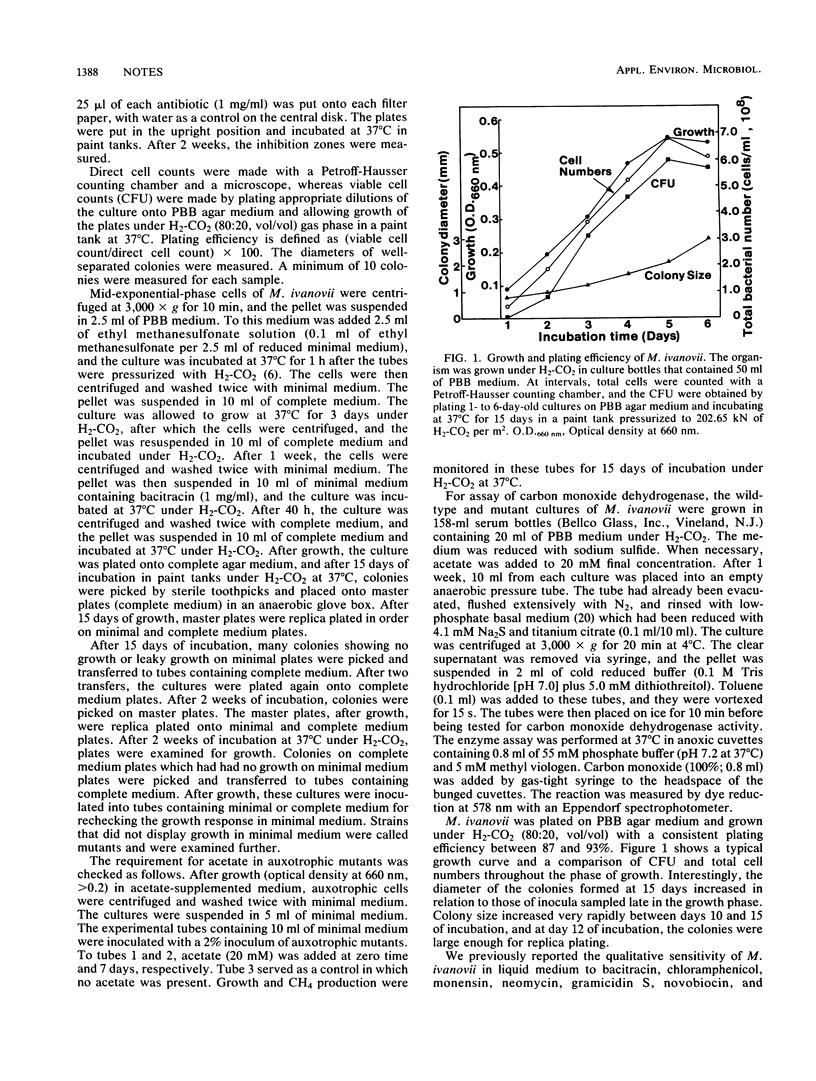
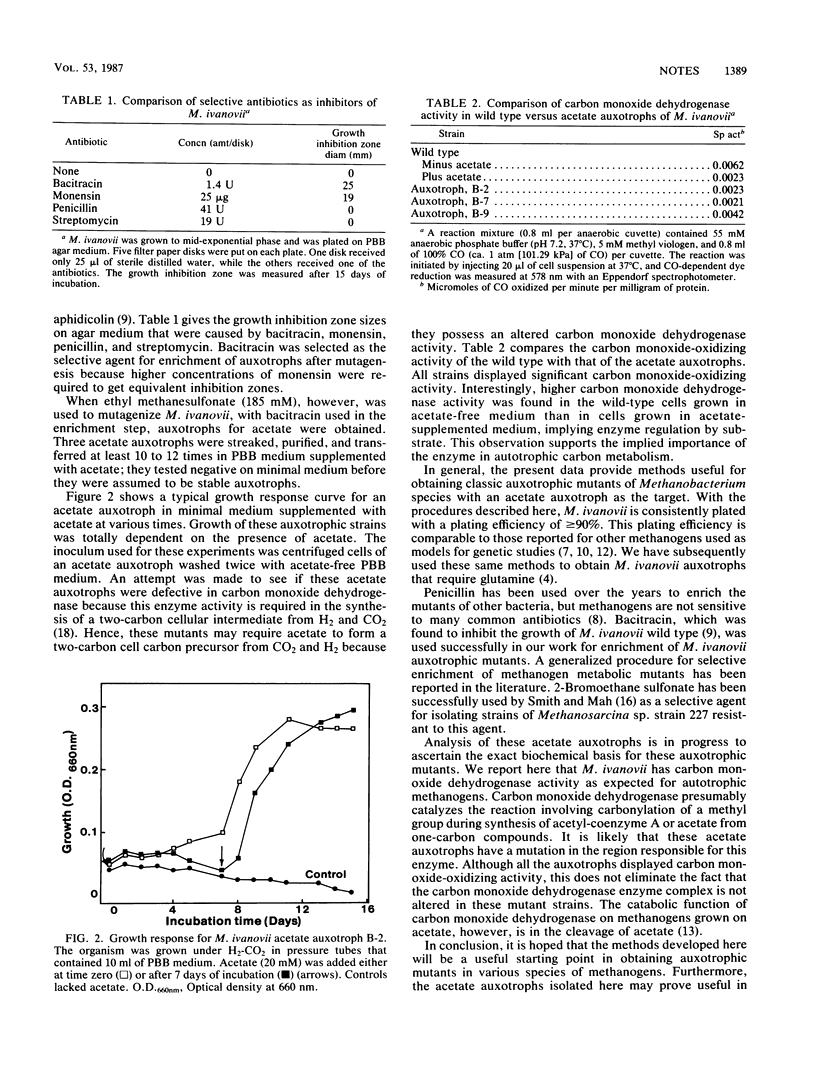
To develop a biochemical genetic approach to understanding cell carbon synthesis or metabolic pathways in methanogens, Methanobacterium ivanovii was selected as a model organism for genetic manipulation studies. The organism displayed a colony size of 3 to 6 mm in less than 2 weeks and had a plating efficiency of about 90%, which made it suitable for replica plating. Mutagenesis and selection techniques were developed for selection of acetate auxotrophs. Chemical mutagenesis with ethyl methanesulfonate, followed by enrichment with bacitracin as a selective agent, resulted in stable acetate auxotrophs. M. ivanovii was very sensitive to UV, but UV-induced acetate auxotrophs were unstable and reverted within two to four transfers. The acetate auxotrophs were analyzed in relation to wild type for carbon monoxide dehydrogenase enzyme activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev S. S., Wolkin R., Kenealy W. R., Deniro M. J., Epstein S., Zeikus J. G. Methanogenic bacteria from the bondyuzhskoe oil field: general characterization and analysis of stable-carbon isotopic fractionation. Appl Environ Microbiol. 1983 Feb;45(2):691–697. doi: 10.1128/aem.45.2.691-697.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar L., Jain M. K., Aubert J. P., Zeikus J. G. Comparison of assimilatory organic nitrogen, sulfur, and carbon sources for growth of methanobacterium species. Appl Environ Microbiol. 1984 Oct;48(4):785–790. doi: 10.1128/aem.48.4.785-790.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T., McBride B. C. New method for the isolation and identification of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):540–545. doi: 10.1128/am.29.4.540-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. J., Whitman W. B., Fields R. D., Wolfe R. S. Growth and plating efficiency of methanococci on agar media. Appl Environ Microbiol. 1983 Jul;46(1):220–226. doi: 10.1128/aem.46.1.220-226.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzycki J. A., Zeikus J. G. Characterization and purification of carbon monoxide dehydrogenase from Methanosarcina barkeri. J Bacteriol. 1984 Apr;158(1):231–237. doi: 10.1128/jb.158.1.231-237.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Trun N. J., Hamilton P. T. Beginning genetics with methanogens. Basic Life Sci. 1982;19:233–244. doi: 10.1007/978-1-4684-4142-0_19. [DOI] [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


