Abstract
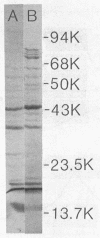
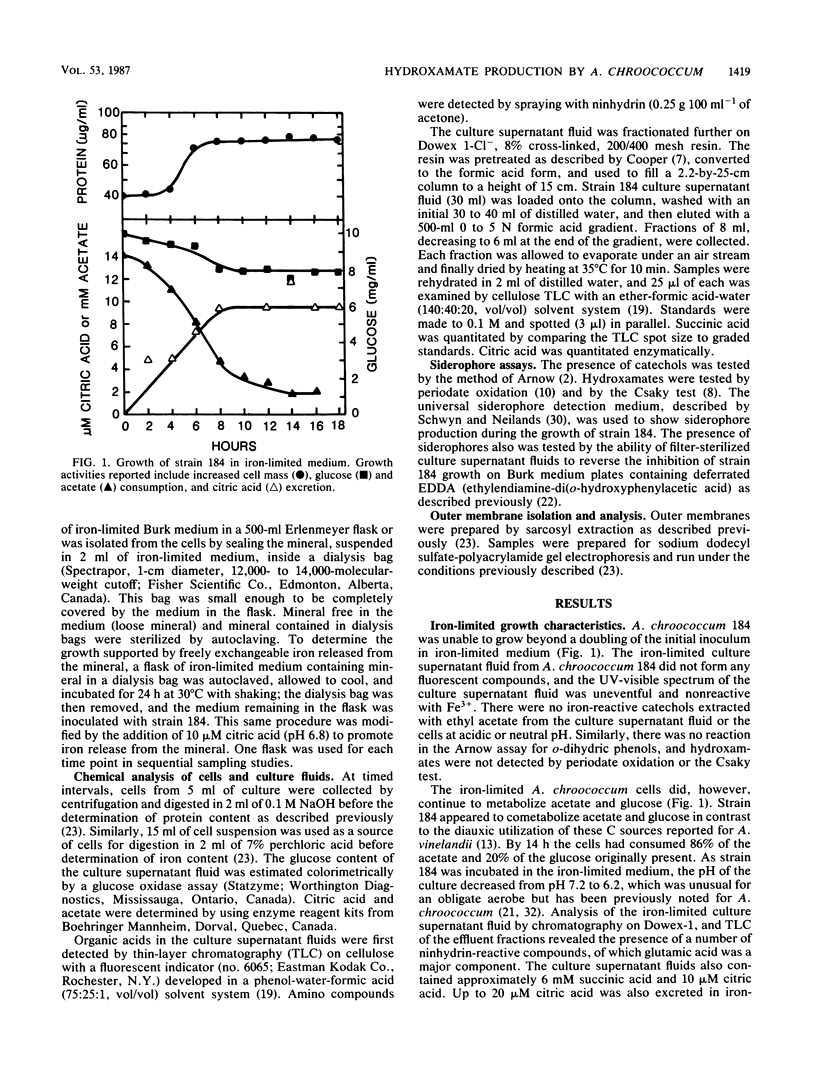
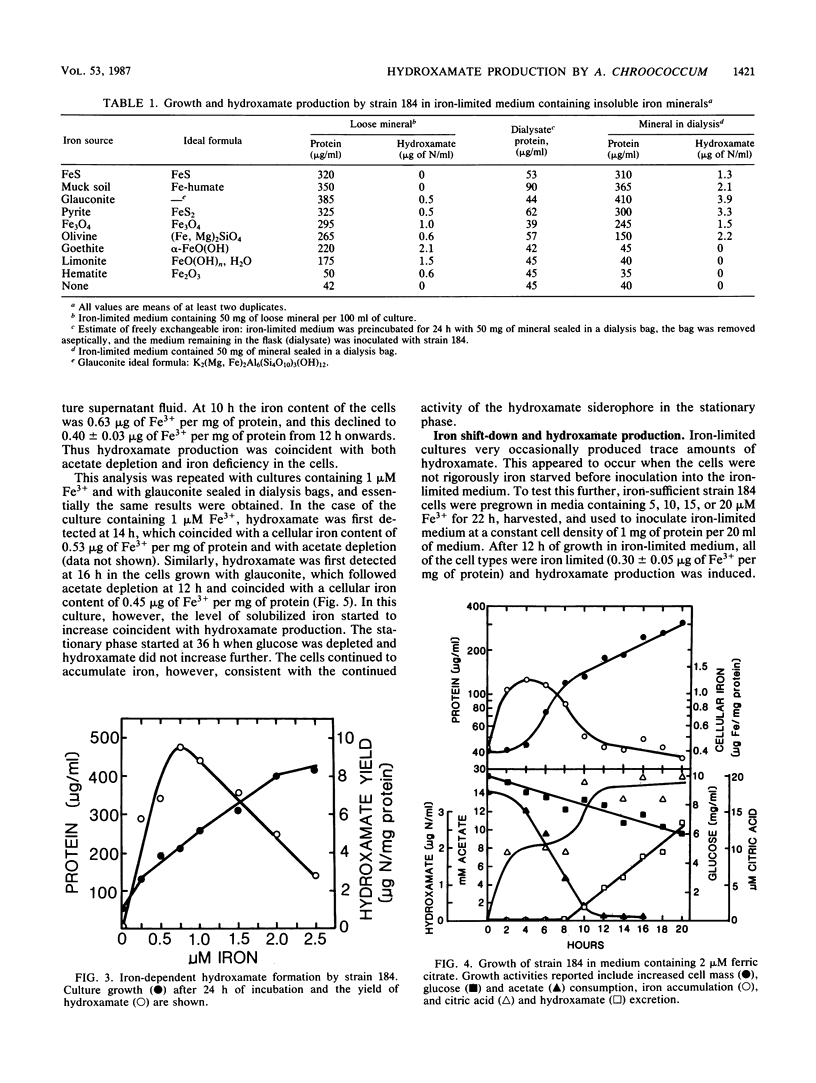
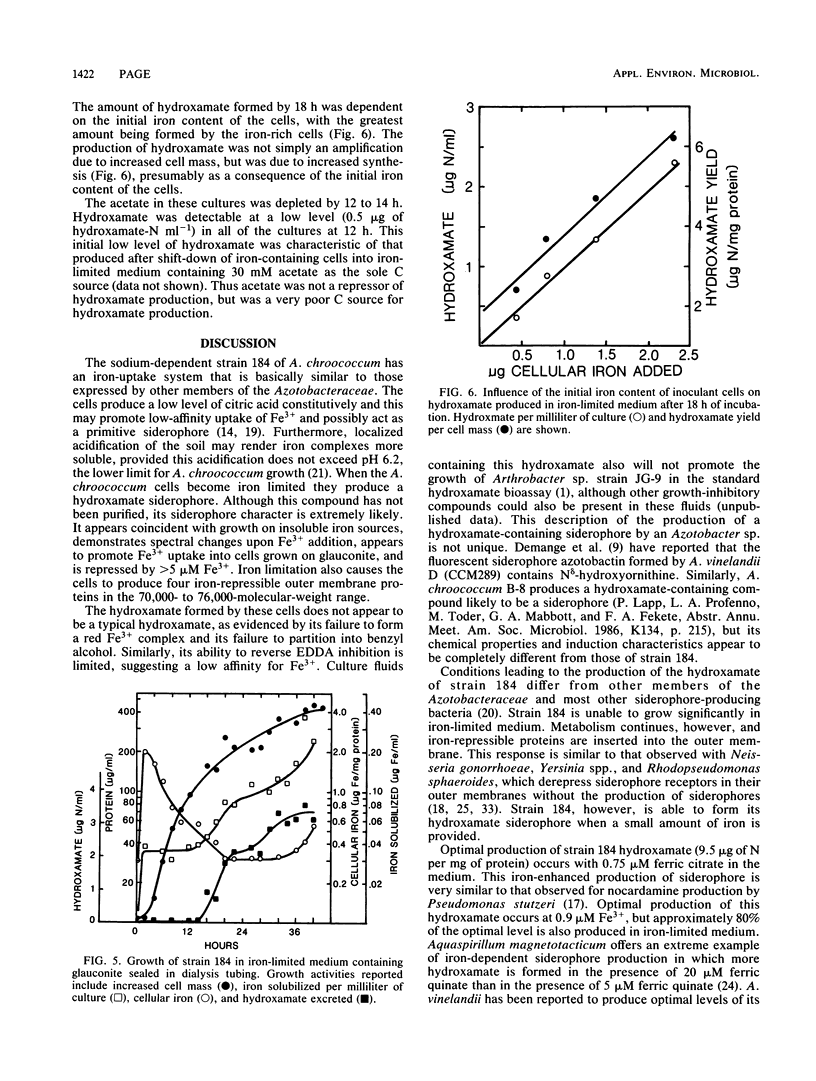
The sodium-dependent strain 184 of Azotobacter chroococcum was unable to grow significantly in iron-limited medium, but did produce iron-repressible outer membrane proteins. Siderophores were not produced under these conditions. Citric acid was excreted, but not in response to iron limitation. This strain, however, was able to grow in insoluble mineral iron sources, and under these conditions the cells produced a hydroxamate. Growth on minerals and hydroxamate production was dependent on a low level of freely exchangeable iron. Optimal hydroxamate production was observed with 0.75 μM ferric citrate, and hydroxamate production was repressed by >5 μM iron. Despite this iron requirement, hyroxamate was only formed during internal iron limitation of the cells. Iron-containing cells were able to grow in iron-limited medium but only produced hydroxamate when their iron-per-cellular-protein content was low. These results, the spectral changes observed upon Fe3+ addition, and iron-uptake coincident with hydroxamate production suggested that the hydroxamate was a siderophore.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTOINE A. D., MORRISON N. E., HANKS J. H. SPECIFICITY OF IMPROVED METHODS FOR MYCOBACTIN BIOASSAY BY ARTHROBACTER TERREGENS. J Bacteriol. 1964 Dec;88:1672–1677. doi: 10.1128/jb.88.6.1672-1677.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete F. A., Spence J. T., Emery T. Siderophores Produced by Nitrogen-Fixing Azotobacter vinelandii OP in Iron-Limited Continuous Culture. Appl Environ Microbiol. 1983 Dec;46(6):1297–1300. doi: 10.1128/aem.46.6.1297-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. E., Costenbader C. J., Melton T. Diauxic growth in Azotobacter vinelandii. J Bacteriol. 1985 Nov;164(2):866–871. doi: 10.1128/jb.164.2.866-871.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein S., Hantke K., Braun V. Citrate-dependent iron transport system in Escherichia coli K-12. Eur J Biochem. 1981 Jul;117(2):431–437. doi: 10.1111/j.1432-1033.1981.tb06357.x. [DOI] [PubMed] [Google Scholar]
- Knosp O., von Tigerstrom M., Page W. J. Siderophore-mediated uptake of iron in Azotobacter vinelandii. J Bacteriol. 1984 Jul;159(1):341–347. doi: 10.1128/jb.159.1.341-347.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Siderophore utilization and iron uptake by Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1984 Oct;234(1):178–186. doi: 10.1016/0003-9861(84)90339-4. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Page W. J., Dale P. L. Stimulation of Agrobacterium tumefaciens Growth by Azotobacter vinelandii Ferrisiderophores. Appl Environ Microbiol. 1986 Feb;51(2):451–454. doi: 10.1128/aem.51.2.451-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Huyer M. Derepression of the Azotobacter vinelandii siderophore system, using iron-containing minerals to limit iron repletion. J Bacteriol. 1984 May;158(2):496–502. doi: 10.1128/jb.158.2.496-502.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J. Sodium-Dependent Growth of Azotobacter chroococcum. Appl Environ Microbiol. 1986 Mar;51(3):510–514. doi: 10.1128/aem.51.3.510-514.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti L. C., Blakemore R. P. Hydroxamate production by Aquaspirillum magnetotacticum. J Bacteriol. 1986 Jul;167(1):73–76. doi: 10.1128/jb.167.1.73-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Accumulation of iron by yersiniae. J Bacteriol. 1979 Mar;137(3):1290–1298. doi: 10.1128/jb.137.3.1290-1298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Schroth M. N., Hancock J. G. Disease-suppressive soil and root-colonizing bacteria. Science. 1982 Jun 25;216(4553):1376–1381. doi: 10.1126/science.216.4553.1376. [DOI] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]