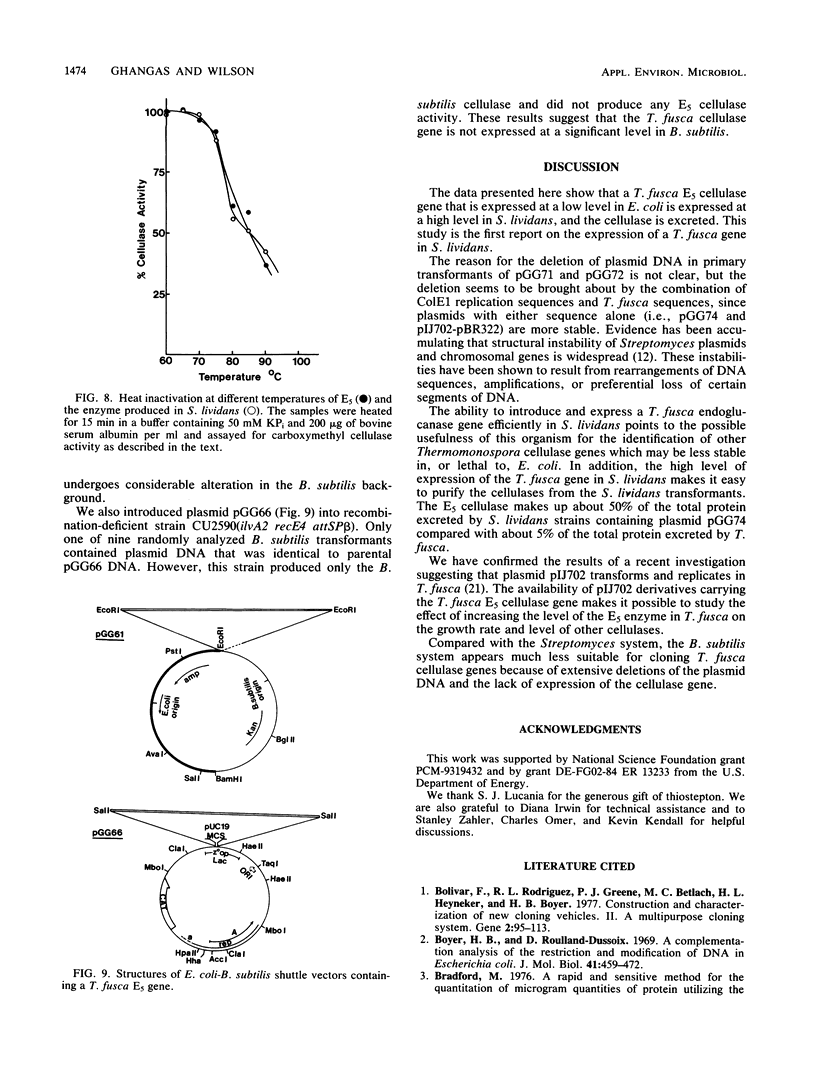
Abstract
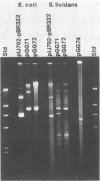
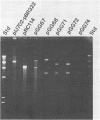
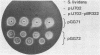
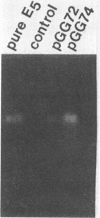
A cellulase gene from Thermomonospora fusca coding for endocellulase E5 was introduced into Streptomyces lividans by using shuttle plasmids that can replicate in either S. lividans or Escherichia coli. Plasmid DNA isolated from E. coli was used to transform S. lividans, selecting for thiostrepton resistance. The transformants expressed and excreted the endocellulase, but the ability to produce the endocellulase was unstable. This instability was shown to result from deletion of the endocellulase gene from the plasmid. Plasmid DNA prepared from a culture in which plasmid modification had occurred was used to transform E. coli, selecting for Amp+ cells, and all of the transformants were cellulase positive, showing that pBR322 and T. fusca DNA were deleted together. When a plasmid was constructed containing only T. fusca DNA in plasmid pIJ702, the transformants were more stable, and the level of endocellulase activity produced in the culture supernatant after growth on 0.2% glucose was close to the level produced by T. fusca cultures grown on 0.2% cellulose. About 50% of the total protein in the culture supernatant of the S. lividans transformant was endocellulase E5. The enzyme produced by the S. lividans transformant was identical to pure T. fusca E5 in its electrophoretic mobility and was completely inhibited by antiserum to E5. Shuttle plasmids containing the E5 gene that could replicate in Bacillus subtilis and E. coli were also constructed and used to transform B. subtilis. Again there was extensive deletion of the plasmid DNA during transformation and growth in B. subtilis. There was no evidence of E5 activity, even in those B. subtilis transformants that retained the E5 gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hägerdal B. G., Ferchak J. D., Pye E. K. Cellulolytic Enzyme System of Thermoactinomyces sp. Grown on Microcrystalline Cellulose. Appl Environ Microbiol. 1978 Oct;36(4):606–612. doi: 10.1128/aem.36.4.606-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackey C. J., Warburg R. J., Halvorson H. O., Zahler S. A. Genetic and physical analysis of the ilvBC-leu region in Bacillus subtilis. Gene. 1984 Dec;32(1-2):49–56. doi: 10.1016/0378-1119(84)90031-3. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pidcock K. A., Montenecourt B. S., Sands J. A. Genetic Recombination and Transformation in Protoplasts of Thermomonospora fusca. Appl Environ Microbiol. 1985 Sep;50(3):693–695. doi: 10.1128/aem.50.3.693-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. Cloning of antibiotic resistance and nutritional genes in streptomycetes. J Bacteriol. 1982 Aug;151(2):668–677. doi: 10.1128/jb.151.2.668-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980 Jul 31;286(5772):525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis: evidence for selective induction of prophage in competent cells. J Bacteriol. 1975 Jan;121(1):296–304. doi: 10.1128/jb.121.1.296-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]